Williamson Ether Synthesis
The Williamson ether synths is, essentially, a simple SN2 reaction. But despite its simplicity, this reaction can easily trick unprepared students and become a determining factor between a passing and a failing grade on the test.

What is the Williamson Ether Synthesis Reaction?
Alexander William Williamson first introduced the ether synthesis that bears his name in 1850. In his pioneering paper, he presented empirical evidence of ether formation under acidic and basic conditions. At that time, the underlying mechanisms of chemical reactions were a mystery, limiting Williamson’s ability to explain his findings. It wasn’t until J.J. Thomson’s discovery of the electron in 1897 that scientists began to understand reactions at the molecular level. Prior to this, Williamson’s empirical data, while quite important for the field of organic chemistry, offered limited predictive insight into the behaviors of the molecules that he observed.
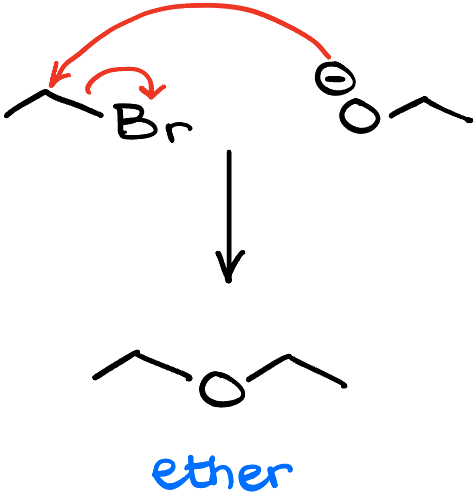
Nowadays, we know that the Williamson ether synthesis is a reaction between an alkoxide and an alkyl halide. Mechanistically speaking, it is an SN2 process. So, for instance, if I were to take 1-bromoethane and treat it with sodium ethoxide, I’ll end up making diethyl ether as a product.
Formation of Alkoxides
The first problem you’re going to face during the test, is the formation of the alkoxides. Typically, we would want to make our alkoxides from the corresponding alcohols. If I peek into my pKa table, I’ll see that the pKa of alcohols is somewhere around 16 to 18. Which means that I need a sufficiently strong base to deprotonate my alcohol completely. If we say that we’re looking for the Keq value somewhere around 103 or greater, this means that the pKaH value for our base needs to be greater than 21. I’m not going to bore you with the intricacies of the calculations here, but this is something that your instructor expects you to know at this point in the course. So, if you’re feeling a little shaky on your acid-base chemistry and corresponding acid-base calculations, make sure you brush it up. I’ll leave all the links in the description below.
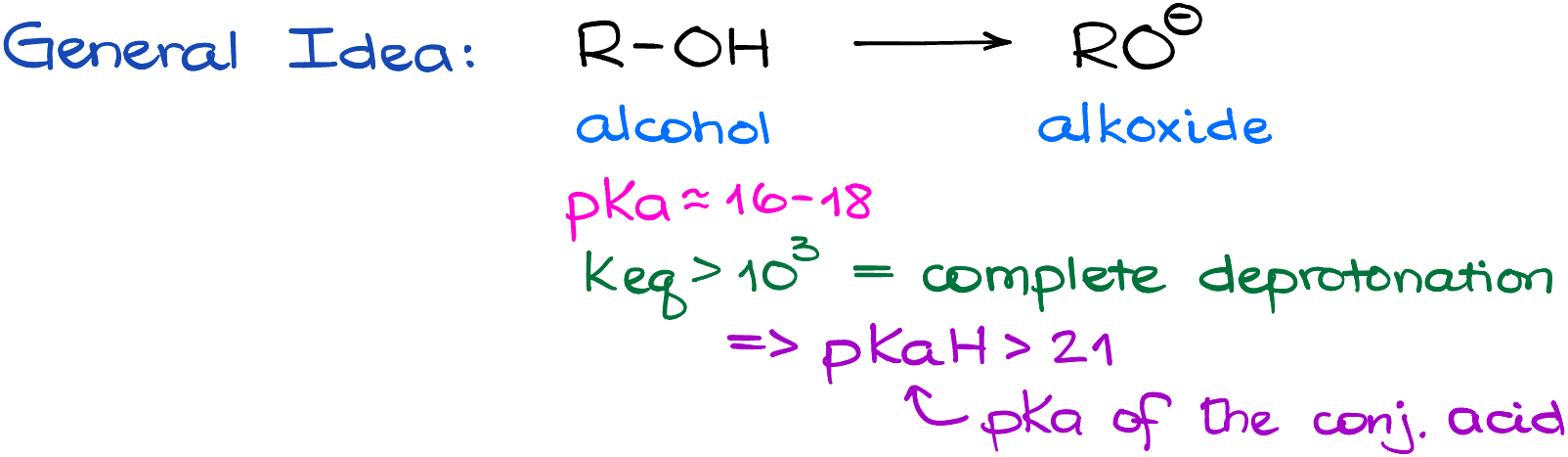
Now, if I consult my pKa table, there aren’t that many bases that would fit the bill in this case. The most common ones are going to be the hydride (H–) and the amide (NH2–) anions. Those, of course, come from the corresponding salts, like sodium hydride (NaH) and sodium amide (NaNH2). For our purposes, the spectator ions like sodium (Na+) are irrelevant. So, it could just as well be lithium (Li+) or potassium (K+), which would make no difference to us.

The most commonly used base is going to be the hydride though. And this is simply because the conjugate acid that we will form in this proton transfer reaction is the hydrogen gas, which quite literally just flies away. This means that we don’t have any unnecessary co-product floating around and potentially messing up our reaction.
Nucleophilic Attack on Alkyl Halide
Once we have our alkoxide, we’re going to add the alkyl halide to our mixture to have those two species react with each other. And since alkoxides are both nucleophiles and bases, we’re going to face certain limitations here.
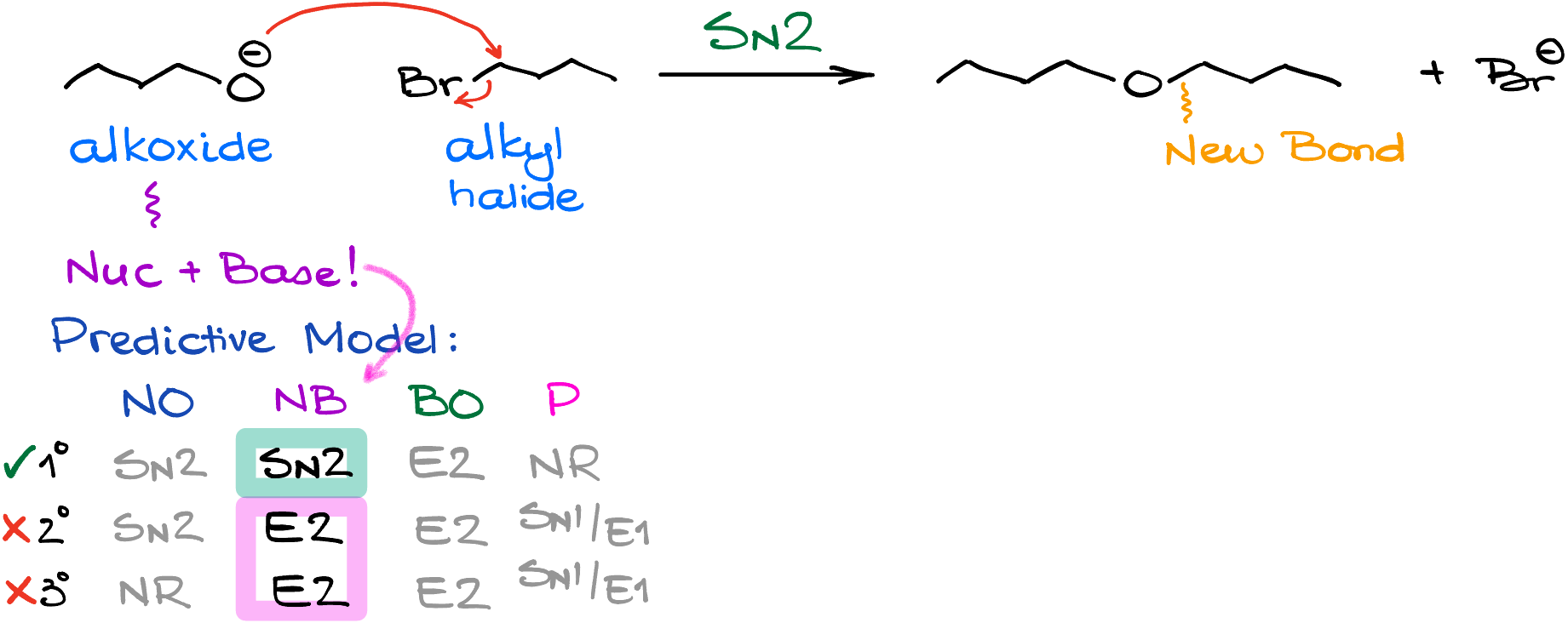
If I go back to my predictive model for the substitution and elimination reactions, we can see that the substitution is only possible at the primary position. If I have a secondary alkyl halide, the substitution product will be a minor product. And when it comes to the tertiary halides, the SN2 reaction there is pretty much impossible. This means, that when you’re planning your synthesis, you’ll be limited to the primary alkyl halides and methyl halides.
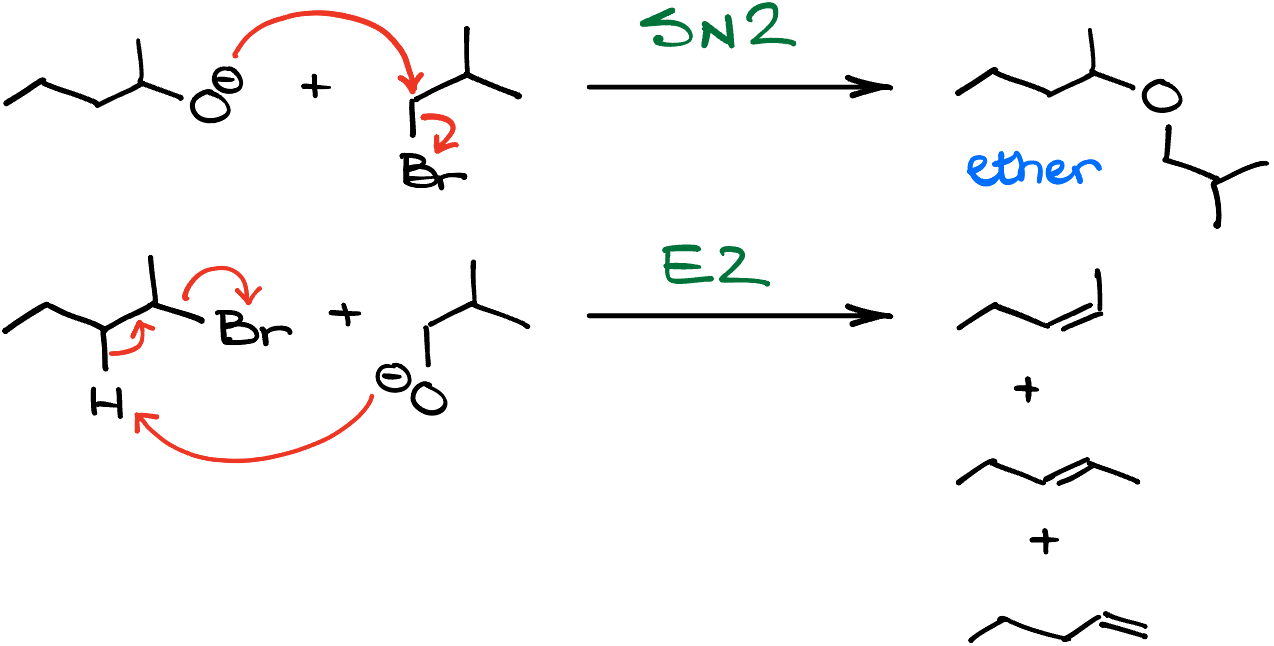
So, for instance, if I react the secondary alkoxide with a primary alkyl halide, I’ll get the corresponding substitution product. But if I reverse the roles and have a secondary alkyl halide as the reagent here, my major product will be an alkene or a mixture of alkenes depending on the structure of the halide!
Planning Williamson Ether Synthesis
The limitation that I described a moment ago is what you’re going to be tested on when it comes to this topic. So, the typical exam question will offer you some sort of an ether and then you’ll either have to come up with the reagents yourself, or you’ll be given a few combinations to choose from in the multiple-choice question style.
So, for instance, let’s say you need to synthesis this butoxybenzene. There are two possible approaches to this synthesis. One approach would be to react phenoxide anion with primary butyl halide, like 1-bromobutane. While the other option would be to react bromobenzene with primary butoxide.
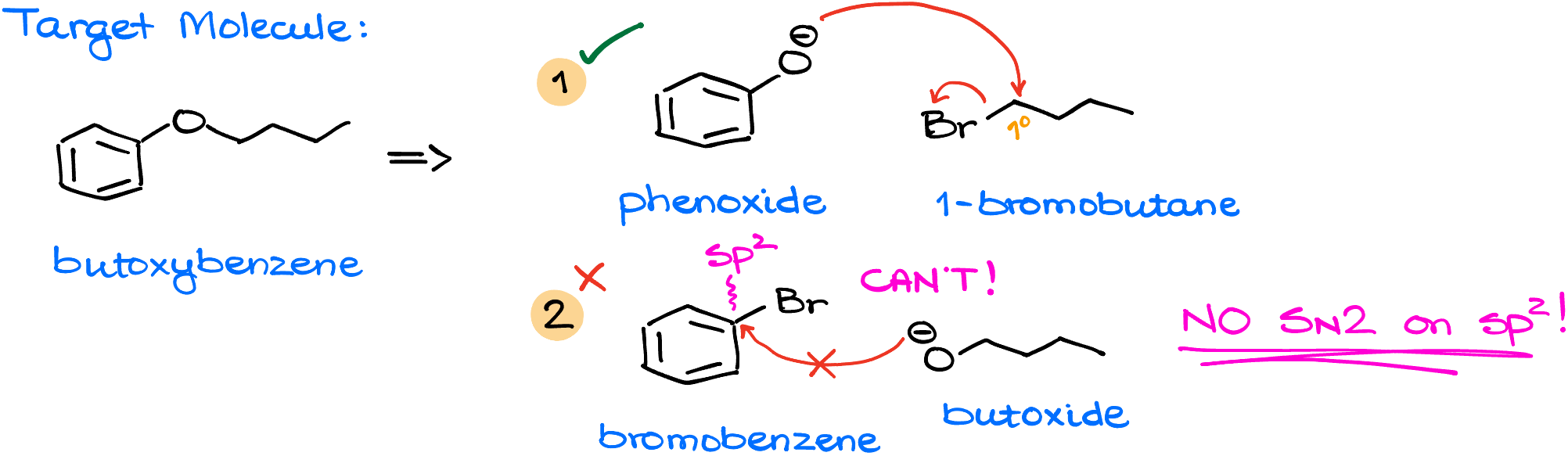
In this case the first approach works without any problems. Phenoxide acts as a nucleophile and easily replaces the bromine leaving group in our alkyl halide. In the second case, however, we have a problem—it’s impossible to have a substitution on the sp2-hybridized atom via a simple SN2 mechanism. Since SN2 requires a back-side attack, it’s physically impossible to do it in this case as the attack would have to originate from the middle of the aromatic ring. Remember that mnemonics I taught you in the SN2 video? No SN2 on sp2! This means that the only option for us is the first combination of reagents.
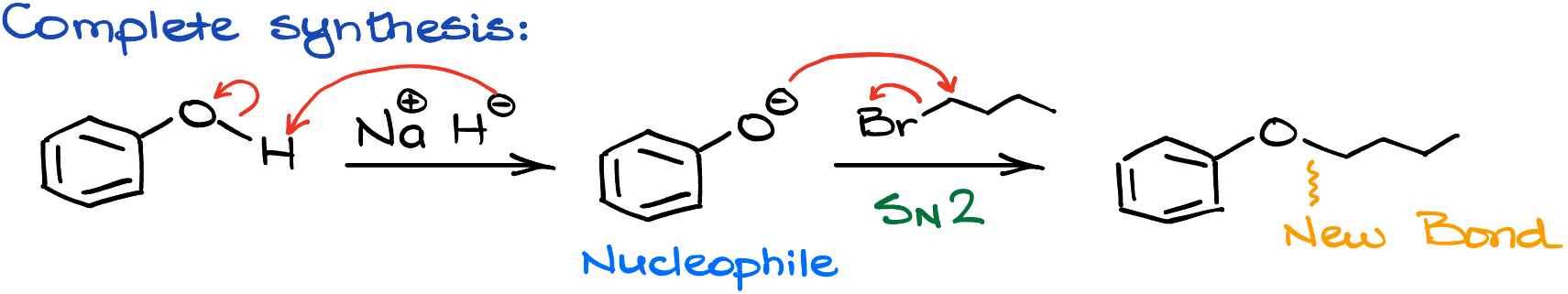
This way, if I wanted to propose the synthesis, I would start with a corresponding alcohol—phenol in this case. I would deprotonate it with sodium hydride (NaH). It is a bit of an overkill in this case, since phenols are fairly acidic in comparison to regular alcohols, but let’s not break a common pattern that we’re learning here. Then, once I have my nucleophile, I’ll introduce the electrophile into the system. In this case, it’s bromobutane. And the resulting SN2 reaction gives me my target molecule.
While this is a fairly simple example, I still want to point out that you need to pay close attention to which atoms you’re connecting and in what order. It’s easy to lose carbons or add a few here and there when you’re disconnecting your molecules like this. And it is a common mistake too. So, if you tend to lose your atoms or add additional ones, it might be a good idea if you number your carbons while working through your reactions.
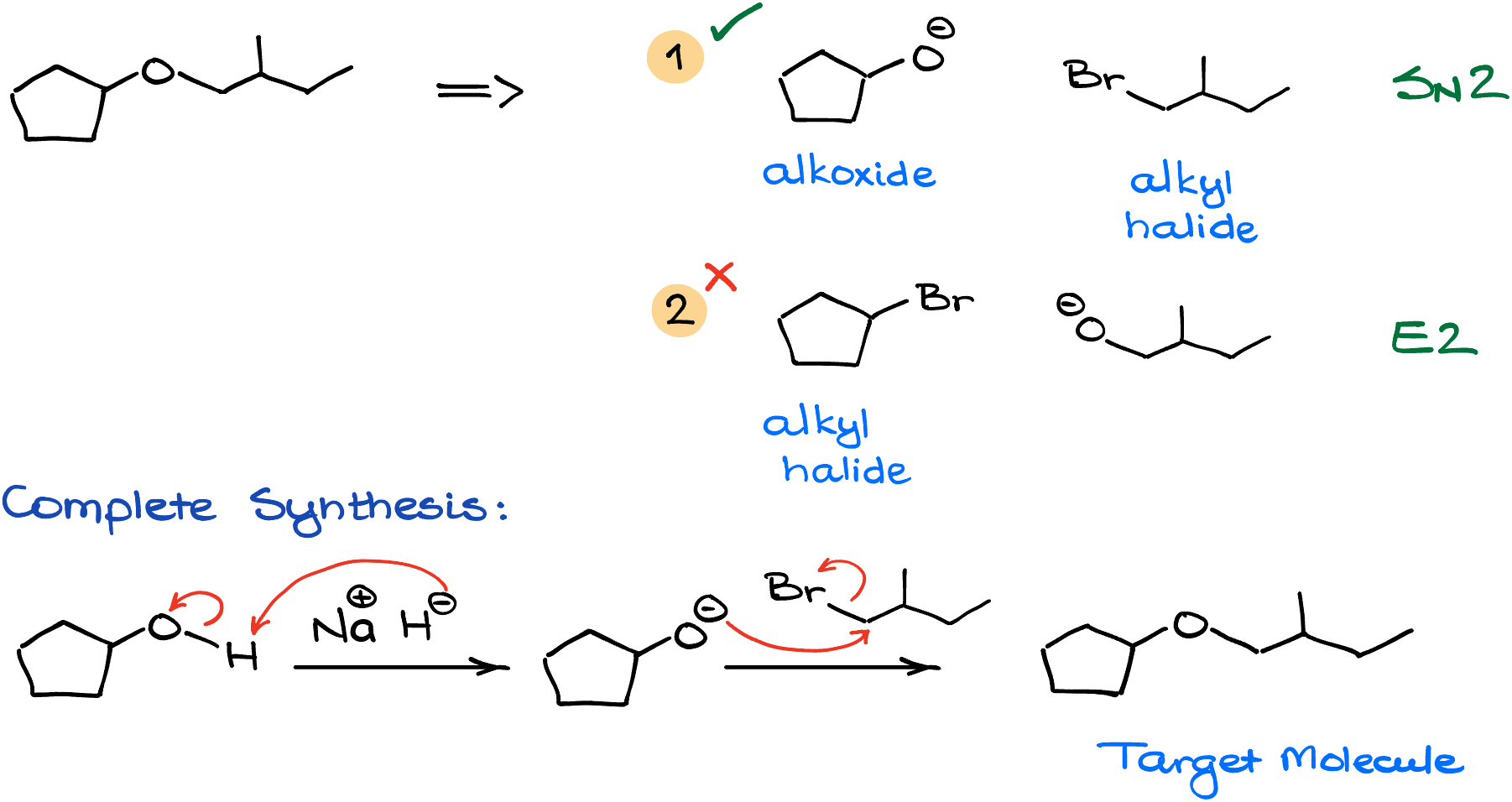
For instance, let’s look at the next target molecule. In this case, we again have two possible options for our reagents. One, in which I have a cyclic alkoxide plus a primary alkyl halide. And the other one, where I have a cyclic alkyl halide and a primary alkoxide.
So, looking at these two combinations, I’ll go with the first combo again as it will be more likely to give the SN2 product than the second combination of my reagents. And of course, to complete the synthesis, I would start with the corresponding alcohol, deprotonate it with sodium hydride, and react it with the alkyl halide to get the target molecule.
Intramolecular Williamson Ether Synthesis
Another interesting aspect of this reaction is that the Williamson ether synthesis can be done intramolecularly. In other words, we can make cyclic ethers using this reaction.
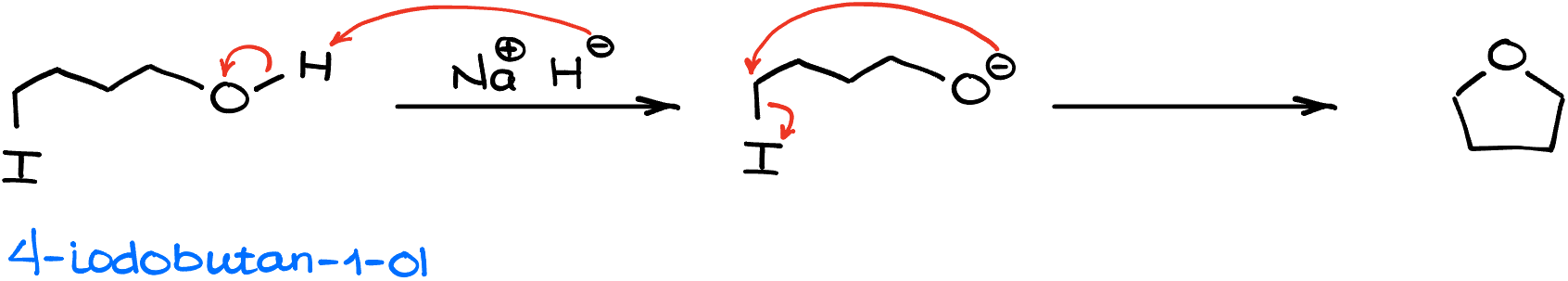
For instance, if I have 4-iodobutane-1-ol and I treat it with sodium hydride (NaH), I would initially deprotonate my alcohol. Then, the resulting alkoxide will react with itself replacing the iodine in the intramolecular SN2 reaction. This yields a 5-membered ring with an oxygen atom in it.

Another common intramolecular version of this reaction is the epoxide formation. Like, for instance in this reaction. Here, oxygen attacks the carbon with the leaving group making a 3-membered ring.
And since organic chemists love molecules so much, we try to put a ring on them all the time. So, questions like these are not only possible on the test, but are quite likely. Remember, that if you can make a 3, 5, or a 6-membered ring via the Williamson ether synthesis, go for it! The 4-membered rings, however, don’t tend to form easily. And as those are going to be unlikely products, steer clear of those guys.
Concluding Thoughts
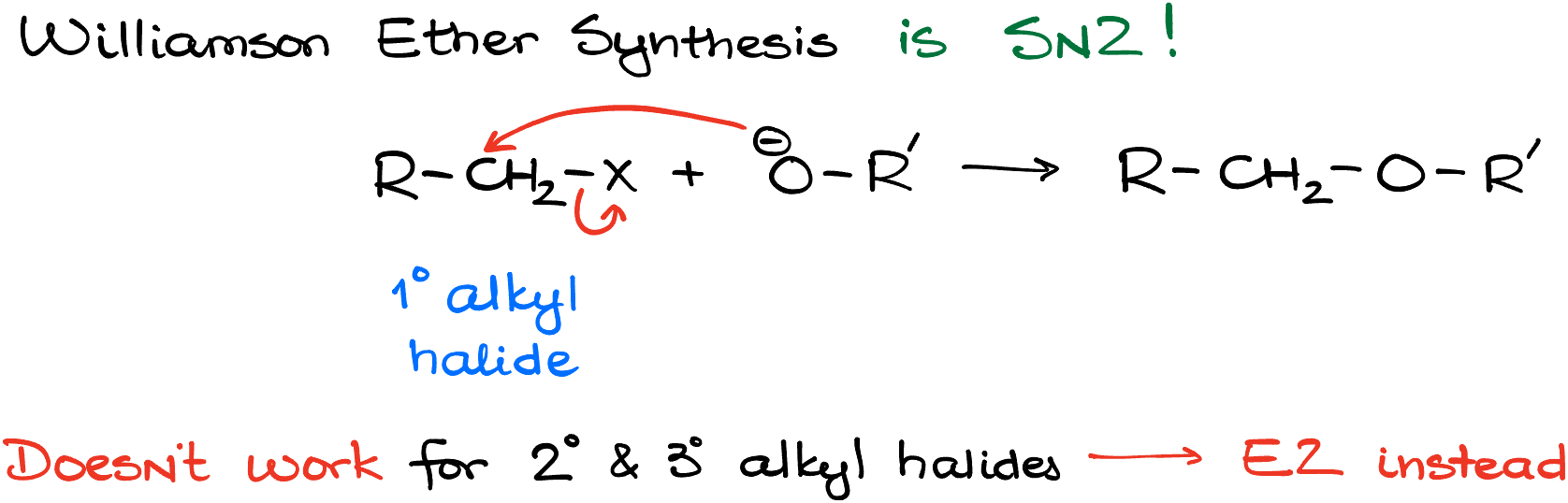
So, to recap, remember that the Williamson ether synthesis is an SN2 reaction that works best with primary alkyl halides or methyl halides. If you have a secondary or a tertiary alkyl halide you’re most likely going to end up with the elimination product. Be mindful of the intramolecular reactions (aka, cyclization reactions) and count your carbons carefully!
