What is Aromatic?
In this tutorial I want to talk about the aromatic compounds and the phenomenon of aromaticity. So, what is aromatic? Hint: it has nothing to do with the smell. As the matter of fact, many aromatic compounds actually do have quite an unpleasant smell.
Criteria of Aromaticity
So, to qualify as an aromatic compound, the molecule needs to fit the following four criteria:
- First, the molecule must be cyclic. Which means, we’re looking for molecules with rings in them.
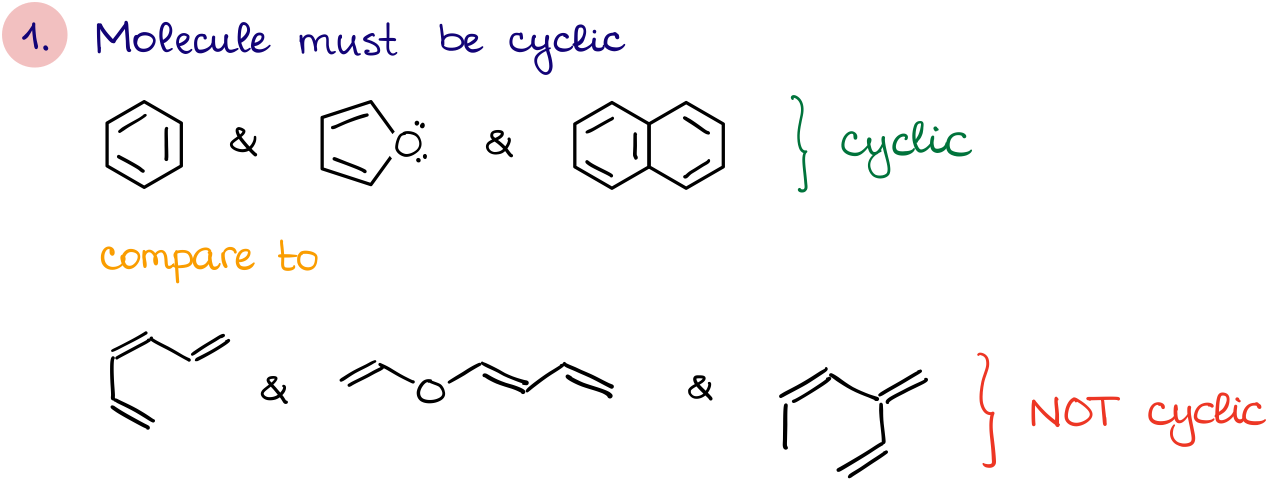
- The molecule must be planar. Or, in other words, the molecule must be flat.

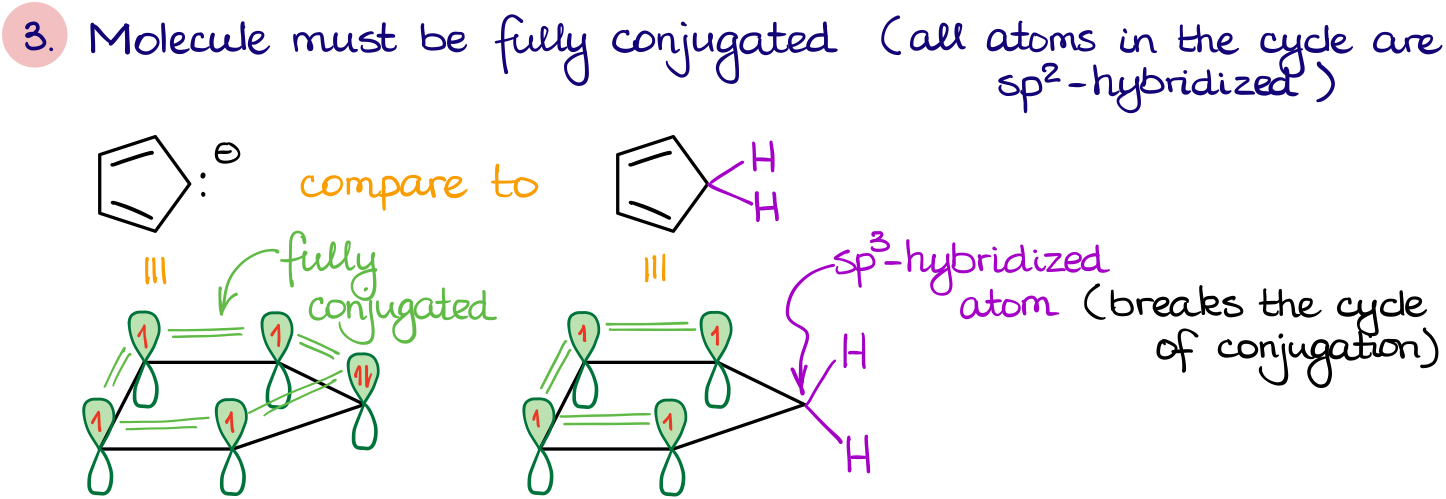
- The molecule also must be fully conjugated. This means that each atom in the cycle needs to be able to participate in resonance. Thus, each atom must be either sp2 or sp hybridized. These two requirements are intertwined. If the cycle is fully conjugated it will always be planar.
- Finally, the last rule is all about the number of electrons in the π-system. This rule is also known as the Hückel’s rule named after Erich Hückel, the German theoretical physicist who’s made a large contribution to the molecular orbital theory of the conjugated π-systems.
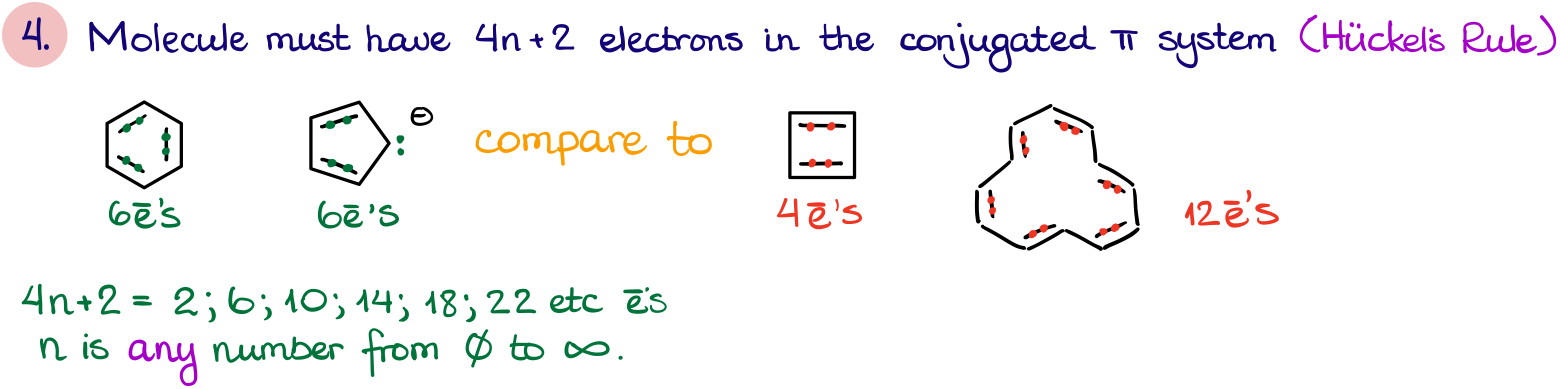
The number of the electrons in the π-system must fit the 4n+2 equation, where “n” is a whole positive real number. Now, here’s something very important you must remember. Repeat after me: the number “n” does NOT correspond to anything in the molecule. So, don’t try to “find” the “n” for a molecule. The rule derived empirically from the molecular orbital theory, so don’t try to make sense of it (at least for now). We’ll talk about the molecular orbitals of aromatic compounds in a different tutorial, so for now it’s just a rule you have to memorize.
For instance, in a benzene molecule we have 6 π-electrons. If we try to solve the equation 4n+2=6 for the “n” term, we get the n=1. So, since we get a whole positive integer, benzene fits the rule.
These are the aromaticity criteria. Thus, if a molecule fits all four criteria, it is aromatic.
I want to emphasize here though that we only include the electrons in the π-bonds and p-orbitals in this calculation. So, all other electrons are completely irrelevant. We’re not going to include the electrons in the σ-bonds, like the C-H bonds, or electrons that are outside of our ring.
So, let’s look a little closer at which electrons we include, and which electrons are not important.
Which Electrons Do We Include in the Count?
As I’ve mentioned, we want to include the electrons in the π-bonds. So, in a case of something like benzene, it’s easy—just count the electrons of the π-bonds, and you’re done.

But this is a simple example, of course. The reality is a little more interesting than that and we’re going to have other types of molecules. For instance, we can have molecules with heteroatoms.
Let’s say we look at these three: pyridine, pyrrole, and thiophene.
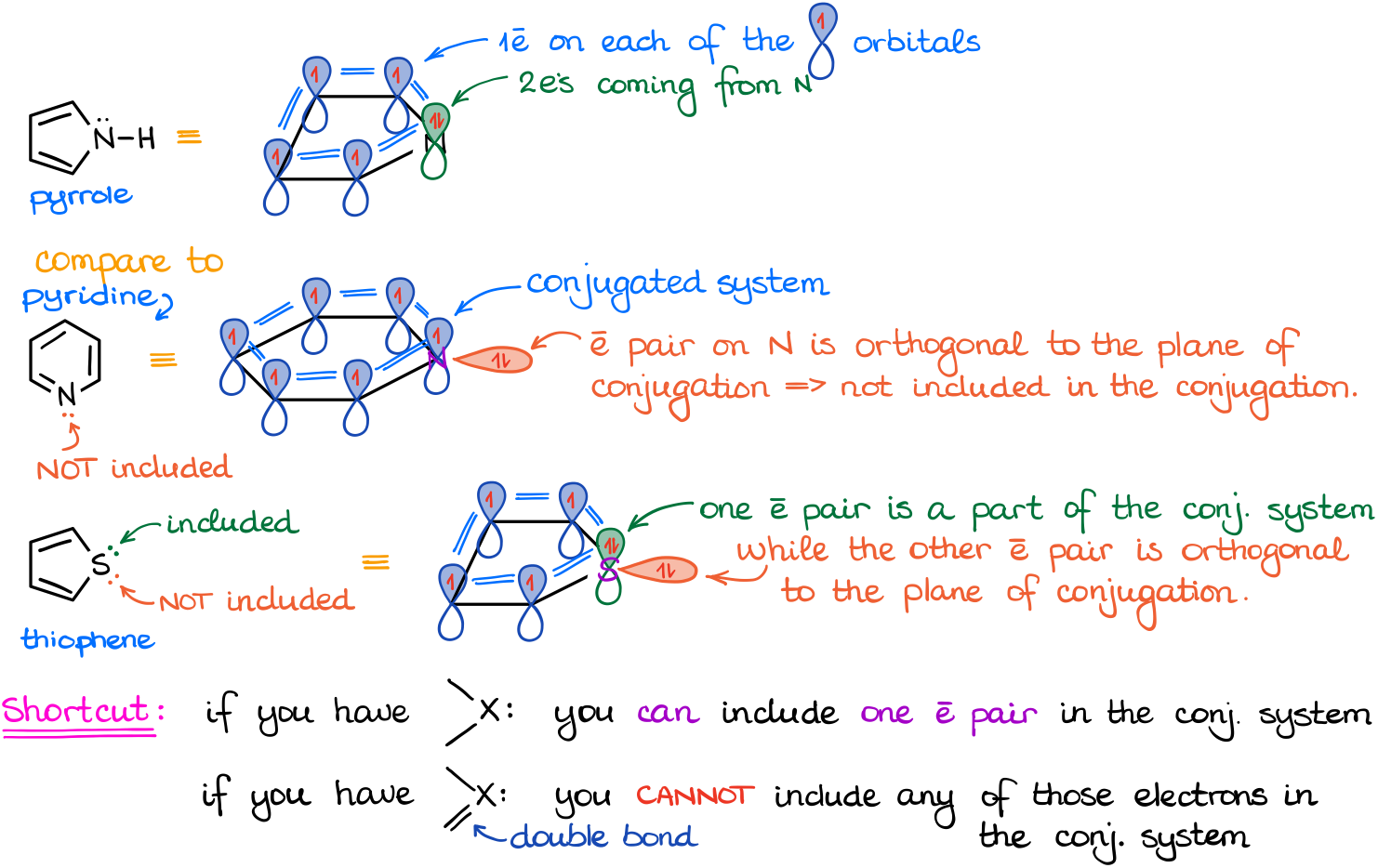
Each of these molecules has π-bonds and the heteroatoms with electron pairs. So, we count the π-bonds, we know that. What are we going to do with the electrons on the heteroatoms though? Do we skip them? Do we include them? And if we do, then do we include all of them or just some? Well, as always, the answer is “it depends” 🤣
Let’s carefully analyze how these molecules look in 3D. And here’s an important observation we can do from these diagrams. If an atom already has a π-bond, like the nitrogen atom in the case of pyridine, we CANNOT use its electrons in the conjugated system and for the purposes of resonance. This is because those electrons are orthogonal (aka perpendicular) to the plane of conjugation. If the element does not have a π-bond, then we can use up to ONE electron pair on that atom. For instance, the nitrogen atom in the pyrrole has one electron pair and since we don’t have a π-bond on that atom, we can put those electrons into the p-orbital and include that in the overall conjugation. However, in the case of the thiophene, the sulfur atom has two electron pairs. Well, since we can only put one electron pair on an orbital, we can only have one of those in the p-orbital which is going to be a part of the conjugated system. Thus, if an element has more than one electron pair, you may ONLY use ONE of those for the purposes of the resonance in the aromatic compounds.
This way, the electrons on the nitrogen atom in pyridine are NOT included. We call those electrons “localized” as they are localized on the nitrogen and are not participating in the resonance.
Electrons on the nitrogen atom in pyrrole ARE included. We call those “delocalized” as they are a part of the resonance conjugation that we have in this molecule.
Finally, in the case of the sulfur in thiophene, we have one localized and one delocalized electron pair. So, we’ll include one of those and we won’t include the other one.
Thus, each of these three molecules has 6 electrons in the π-system. And just like in the case of benzene, they all fit the Hückel’s rule.
Aromatic Ions
We can also see cases in which our atoms in the ring are charged. For instance, here I have the cyclopentadienyl anion, and tropylium cation. We’ll treat the carbanions exactly like the heteroatoms. Thus, in this case we are going to include the electron pair since the carbon with the negative charge does not have a π-bond.
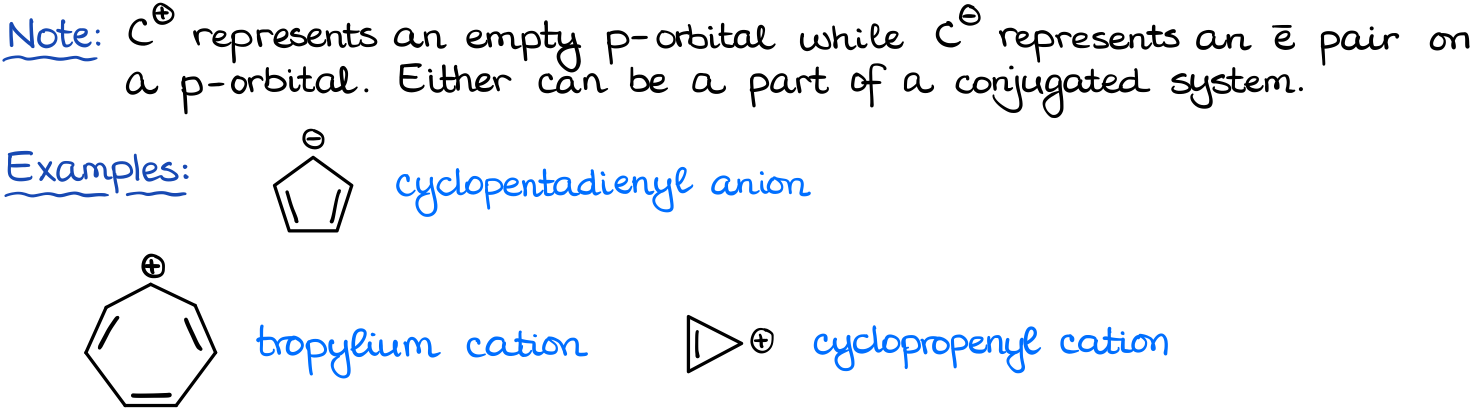
In the case of the carbocations, a charge is the result of the empty p-orbital. And since that empty p-orbital is next to a π-bond, we can also include it in the resonance. However, since the orbital is empty, we’re not going to be adding any electrons to our count for it.
Thus, what we get is 6 and 6 for these two examples as well.
But what happens if we break any of the rules?
If we break any of the first three rules, the molecule is NON-aromatic. Therefore, it’s really important to check the rules in order. Because, if we break one of the first three rules, then it’s completely irrelevant how many electrons you have in the molecule.
This is a very common mistake I see many students make: they jump right the way into the electron counting and completely ignore the rest of the requirements. Be careful with those. Your instructor absolutely will give you examples on the exam that may have all necessary electrons but break one of the first three rules. And if my years of teaching organic chemistry count for anything, I am willing to bet that a lot of students will get caught by that. Many instructors, however, will tell you that you can assume the planarity. Which means that the requirement #2 will be true for all molecules in the set, or at least we’ll count them as such. In that case you still need to check that the molecule is indeed cyclic and fully conjugated before you do any electron counting.
Also, if your electron count gives you an odd number, which only happens in the case of radicals, it’s also going to be a non-aromatic compound. It’s a fairly rare test example as some classes don’t even cover radicals and their reactions till the end of the second semester. But it is still a fair game if you have already learned about the radicals in your course.
Finally, if we fit all the criteria but the electron count fits the 4n formula instead of the 4n+2 it’s an anti-aromatic compound. Notice, anti-aromatic and non-aromatic are NOT the same thing!
Aromatic compounds exhibit an abnormal stability compared to their open-chain (acyclic) versions. So, being aromatic is awesome. Molecules will typically do whatever it takes to become aromatic or maintain aromaticity. Aromatic conjugation also gives several unique chemical properties that we do not see in other types of molecules.
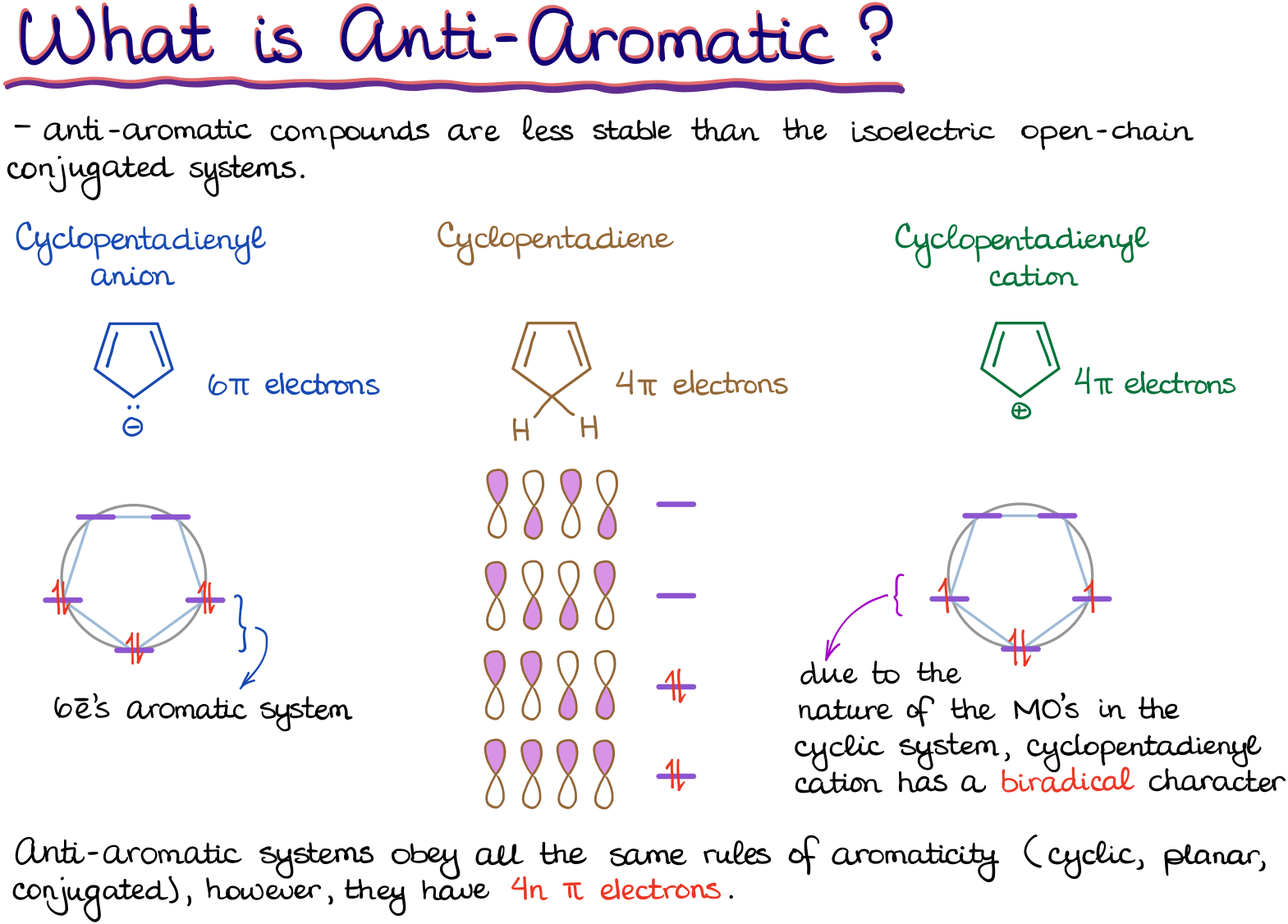
Anti-aromatic compounds are, on contrary, extremely unstable. Thus, being anti-aromatic compound is extremely unfavorable. As the matter of fact, vast majority of anti-aromatic compounds are too unstable to even be isolated and characterized via normal chemical tests. So, molecules will do whatever it takes not to be anti-aromatic.
In the meantime, the non-aromatic compounds… well, those are neither more nor less stable than the open-chain versions of those molecules. In other words, being non-aromatic is nothing special. It’s just a regular molecule. And unlike aromatic compounds, non-aromatic molecules don’t have any special chemical properties either.
