Valence Bond Theory and Lewis Structures
Valence Bond Theory treats bonds as a shared pair of electrons. Each electron supplies one electron to make a bond and those electrons are shared more or less equally by the elements. So, if we look at the simplest molecule possible (H2), we’ll see the following:
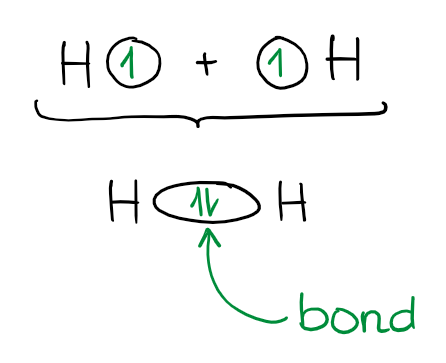
In this example, each hydrogen provides one electron for the bond. And the bond is a shared pair of electrons. Simple enough, right? VBT treats all bond in the same manner.
Lewis Structures
Lewis structures are a simple and the most common way we represent molecules. We show electrons around the atoms with dots:
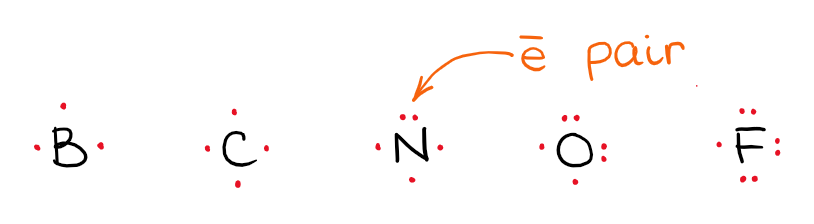
The number of dots equal to the number of valence electrons an element has. The number of valence electrons correspond to the group in which you find the element. Thus, B has 3 valence electrons, C has 4, N has 5, O has 6, and F has 7. You add those electrons onto the atom till you have four electrons. After you get four, you start coupling electrons to make electron pairs. This way, N will have 1 electron pair, O will have 2, and F will have 3.
According to VBT, only the unpaired electrons can participate in bonding. Thus, if we go back to our Lewis structures of the elements above, we can see that:
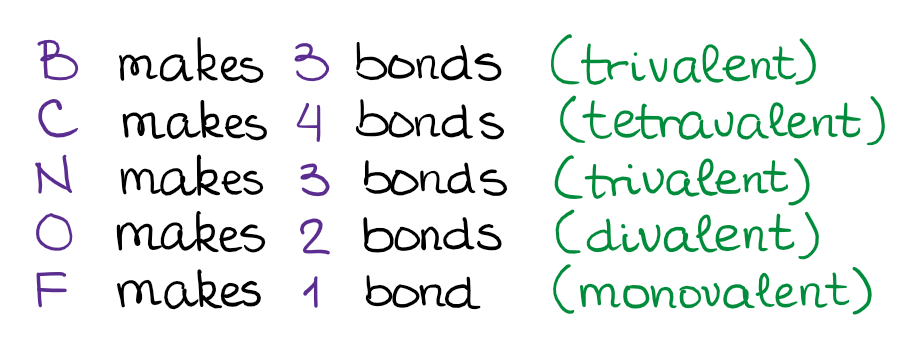
Knowing how many bonds each element typically makes will help you building the molecules out of the elements like legos building blocks!
Bonding Patterns in Neutral Organic Molecules
So, using the valency of the elements from above, we can make the following building blocks:
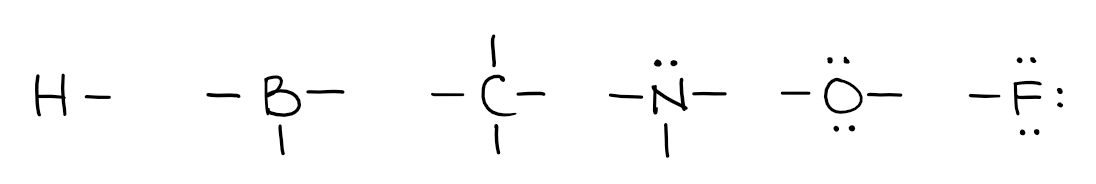
Each line represents a bond. Remember, that each bond is 2 electrons, so to make a bond, the other element needs to provide an electron as well. Let’s look at a few examples:
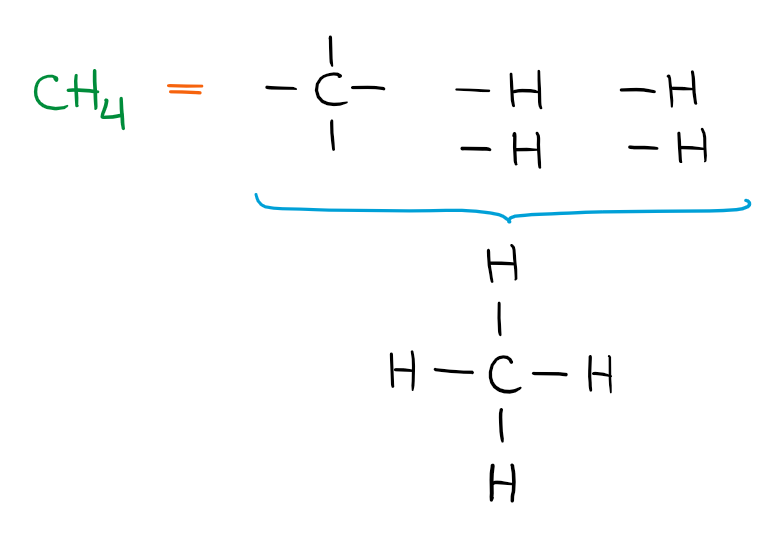
In this molecule, we have five building blocks. The only way to make a molecule out of those building blocks is to have the carbon in the middle and have the four hydrogens attached to it. If we add an extra atom into our molecule, we get the following building blocks:
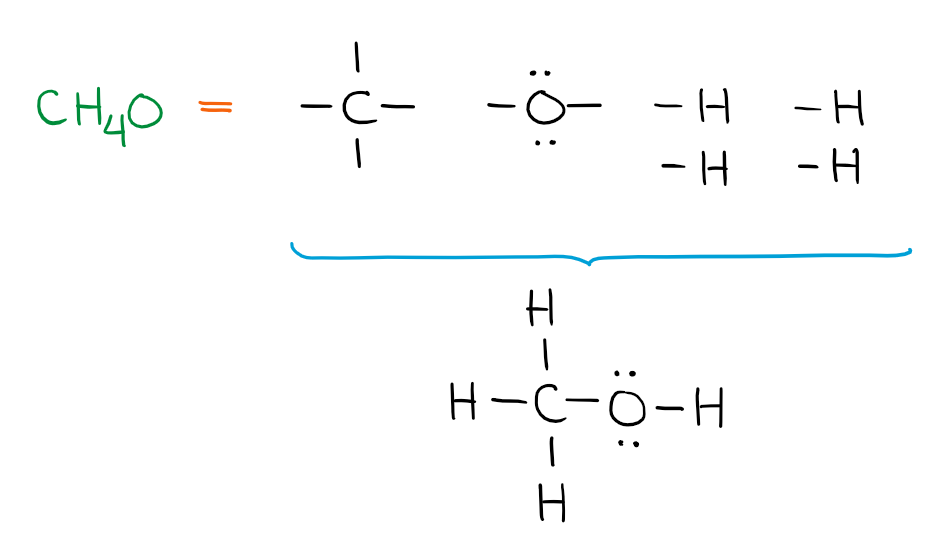
In this case, again, there’s only one way you can build a molecule from these building blocks. HOwever, if you start adding more atoms, you run into a situation when you can make more than one structure from your building blocks:
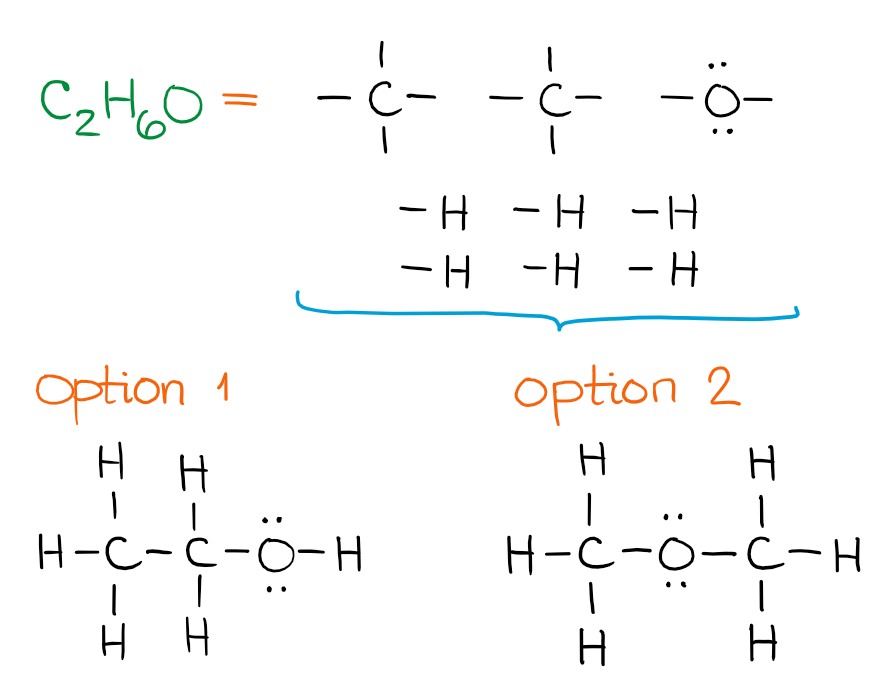
The two options you get for this combination of atoms, are called constitutional isomers. Constitutional isomers have different structure, names, and properties. However, they do have the same molecular formula.
Lewis Structures with Double & Triple Bonds
When you put your building blocks together but still have unconnected bonds, you can make double or triple bonds or even cyclic compounds. For instance, let’s look at the following molecule:
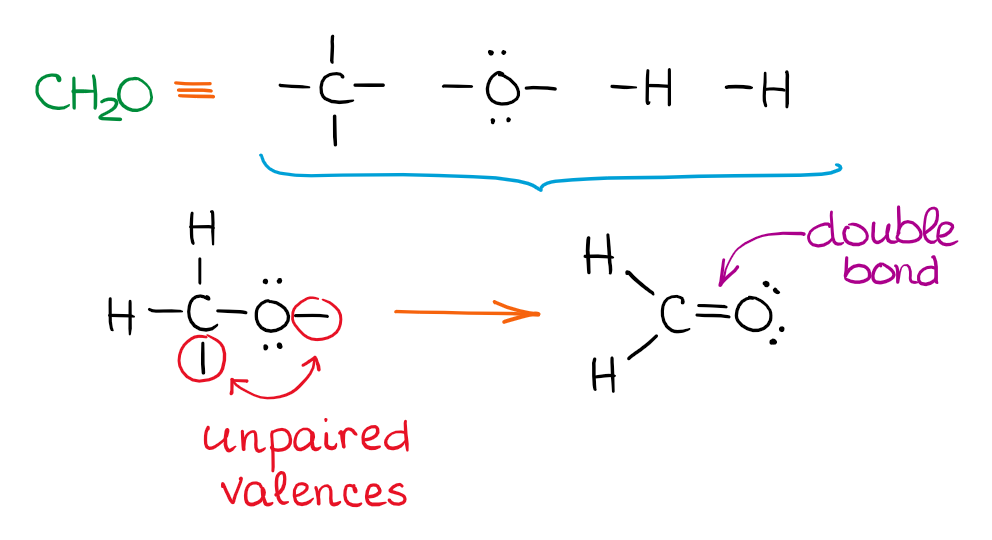
In this case, you have 2 unconnected bonds, so we’ll connect them together to make a C=O bond. The following two molecules are examples of molecules with a carbon-carbon double and triple bond:
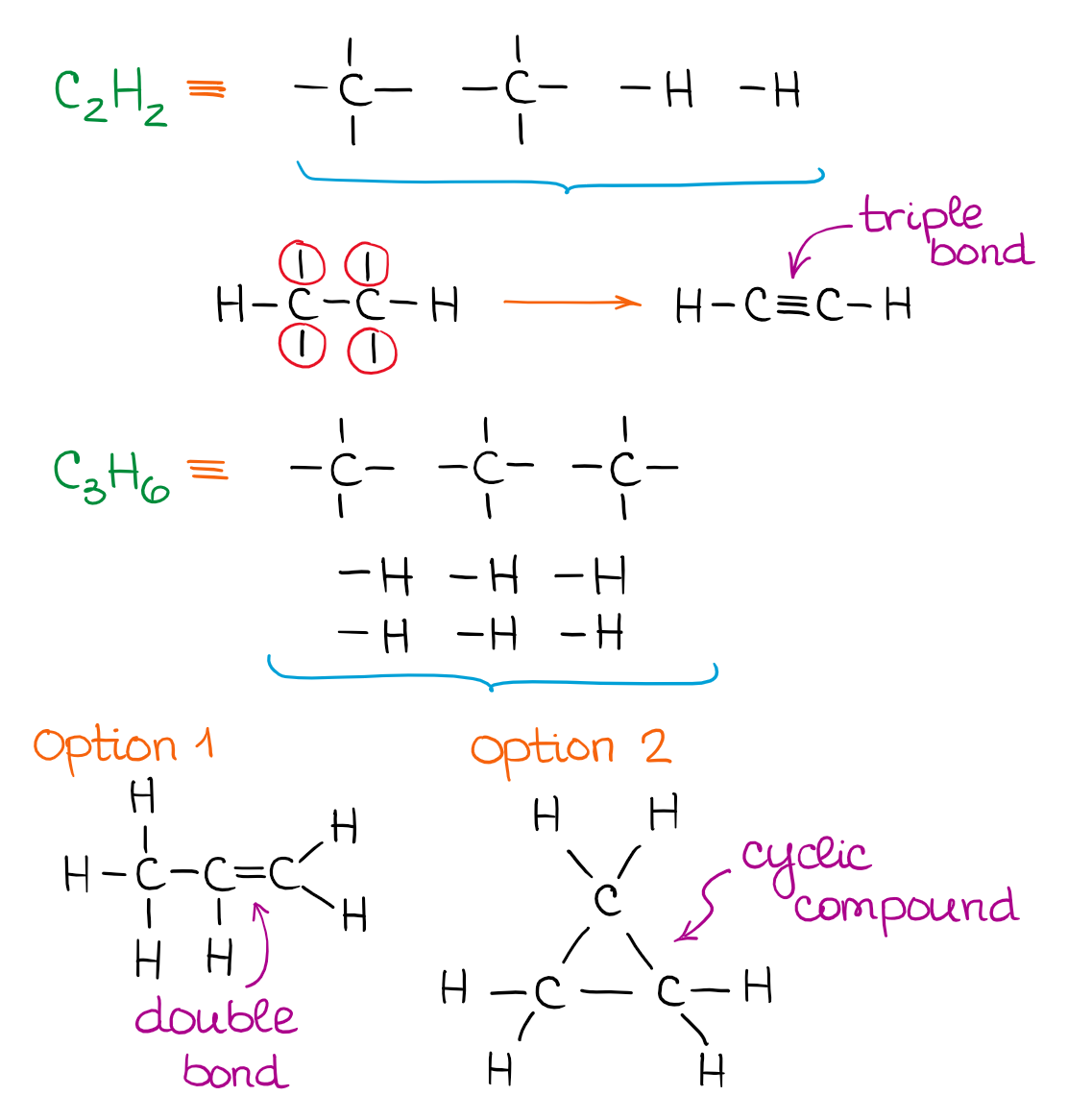
The more atoms you have in the molecule, the more possible constitutional isomers you can get. This number grows exponentially! So once we have a dozen atoms in the molecule, you’re looking at thousands of possibilities!
Limitations of the Valence Bond Theory
- Simplistic approach
- Doesn’t explain or predict the 3D structure of molecules
- Can’t explain some reactivity patterns

If you wonder why use this method if it’s so simplistic? Well, exactly because it IS simplistic AND it still has a high degree of utility. We don’t always need to use the 3D representations or the orbitals. So, when you can use an easier approach, you should use it 😄