Tricky Epoxidation Mechanism Challenge
I have a fun mechanism for you here! Let’s take a look at what we’re dealing with.
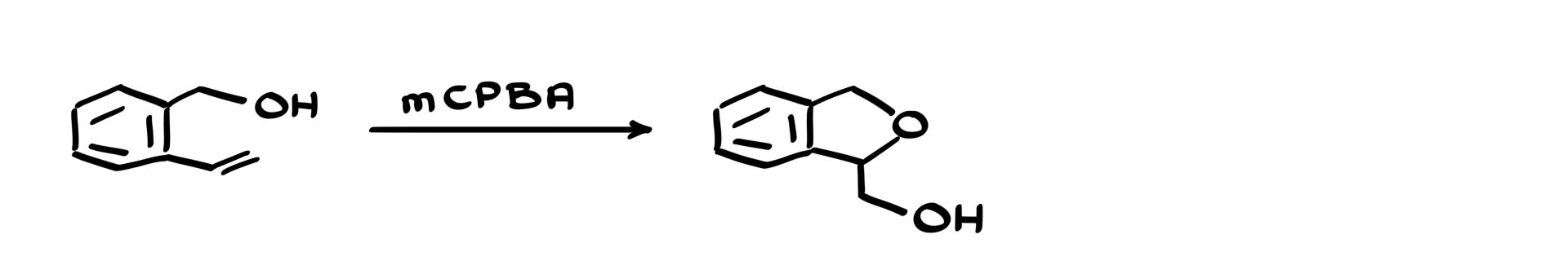
We’re starting with an aromatic molecule that has both an alkene and an alcohol as its functional groups. We’re treating this molecule with mCPBA, which is typically used for epoxidation. But when I look at the final product, it doesn’t look like an epoxide at all! So, let’s walk through the mechanism step by step and figure out what’s happening.
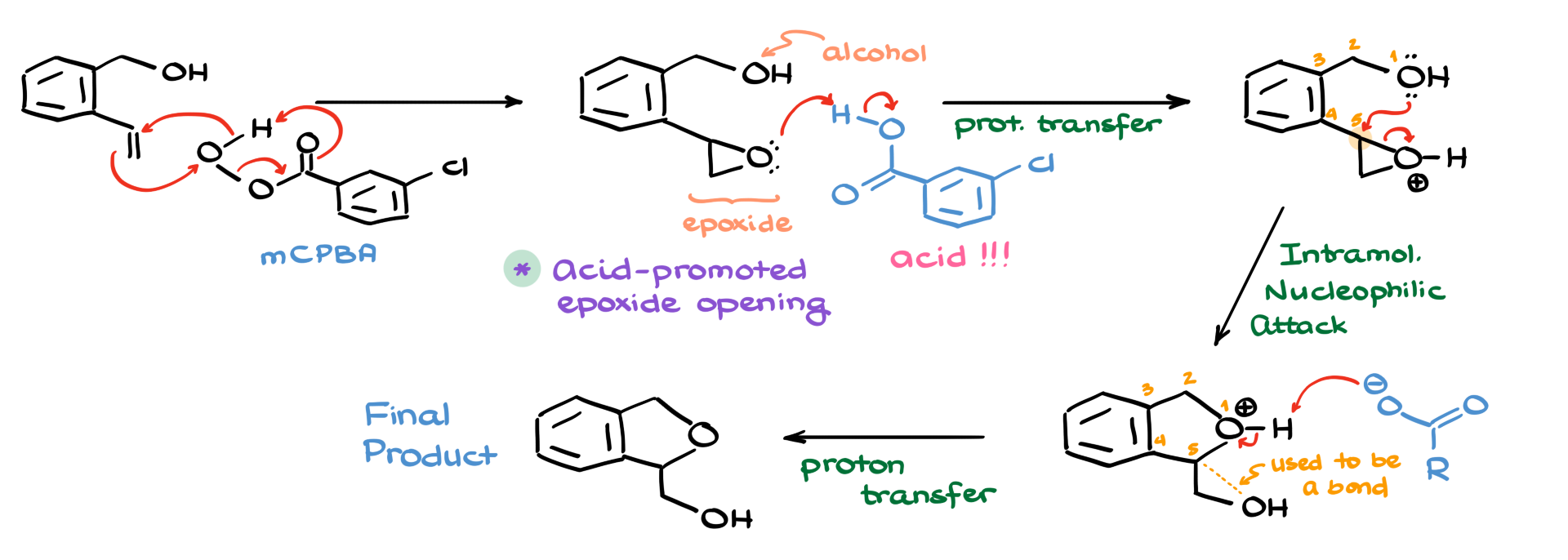
First, I’ll redraw the starting material and show the structure of mCPBA. Since we know this reaction starts with epoxidation, I’ll illustrate the electron movement for this step. There’s also a five-arrow version of this mechanism, so if you prefer that, it works just as well. As expected, the initial outcome of this step is an epoxide.
But here’s something important to keep in mind—the co-product of epoxidation is a carboxylic acid. At the same time, we now have an epoxide and an alcohol in the system, which means we can proceed with an acid-promoted epoxide opening.
So, the next step is protonation of the epoxide. The epoxide grabs a proton from the carboxylic acid, forming a protonated intermediate. From this point, we’re ready for the epoxide opening. Since we are in acidic conditions, epoxides open from the more substituted side. This means our nucleophile—our alcohol—will attack the more substituted carbon of the epoxide, leading to the formation of a five-membered ring.
Now, if we number the atoms in our structure, we can see that oxygen is position 1, followed by carbons 2, 3, 4, and 5. The bond between carbon and oxygen in the epoxide breaks during this process, allowing the formation of the cyclic ether.
The final step in this mechanism involves deprotonation. The conjugate base of the carboxylic acid (the carboxylate ion) removes a proton from the newly formed ring system, yielding our final product.
Pretty cool, right? This mechanism is a great example of how reactions don’t always stop at the expected intermediate. Even though we initially formed an epoxide, the reaction didn’t stop there—it continued to give us a five-membered ring instead.
So, what did you think about this mechanism? Let me know in the comments below!
