The Wittig Reaction
The Wittig reaction, named after Georg Wittig (1979 Nobel Prize in Chemistry), is probably one of the most important carbon-carbon double bond formation reactions you’re going to cover in your class. You’re going to use it in your homework, you’re going to need it for your synthesis problems, and you’re definitely going to see it on the test. So, let’s take a close look at this reaction and talk about everything you’ll need to know for the test.
General Scheme of the Wittig Reaction
In a nutshell, the Wittig reaction is an interaction between a carbonyl such as an aldehyde or a ketone with a phosphoylide giving an alkene.

This reaction can potentially give you either E- or Z-alkene and we also form triphenylphosphine oxide as a co-product.
Mechanism of the Wittig Reaction
Synthetically, we can break down this reaction into two phases. In the first phase, we’re going to prepare the phosphoylide.
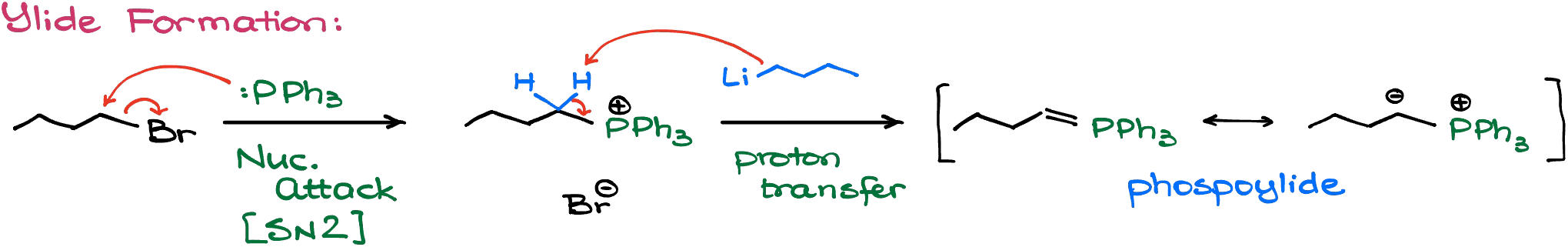
Here, we’re going to start with an alkyl halide. Typically, we’re going to see a 1° alkyl halide. We’re going to treat this alkyl halide with triphenylphosphine. Since phosphines are excellent nucleophiles, we’re going to see a substitution reaction here, making a charged salt with our halide being a counter ion.
This salt is, however, not our final goal. So, we’ll continue by treating it with a very strong base. Typically, we’re going to use butyl lithium. However, you might occasionally see other bases like sodium or potassium hydrides, or even something like LDA.
The reaction with the base is going to result in the formation of the phosphoylide. There are two resonance forms that we can use to represent this species. One has a C=P double bond, while the other one — charges. Which one is more correct is a good question. From the experimental data we know that the nature of the carbon-phosphorous bond here depends on the nature of the ylide itself. So, I don’t wanna make any blanket statements here. In any case, many instructors like to use either or, so pay close attention to how your instructor writes it in class and just go with that representation.
Depending on the nature of the ylide, it may or may not be commercially available. Some ylides are quite reactive and cannot be stored for any appreciable amount of time, and need to be used immediately upon synthesis. Some, on the other hand, are quite stable and can sit on a shelf for decades! Depending on the problem that you’re working on, you may or may not need to show the ylide formation. If your instructor doesn’t specifically ask for the ylide formation in your synthesis scheme, you might as well skip that part and move to the reaction itself, which I describe below.
Once we have our ylide, we’re going to react it with our carbonyl.
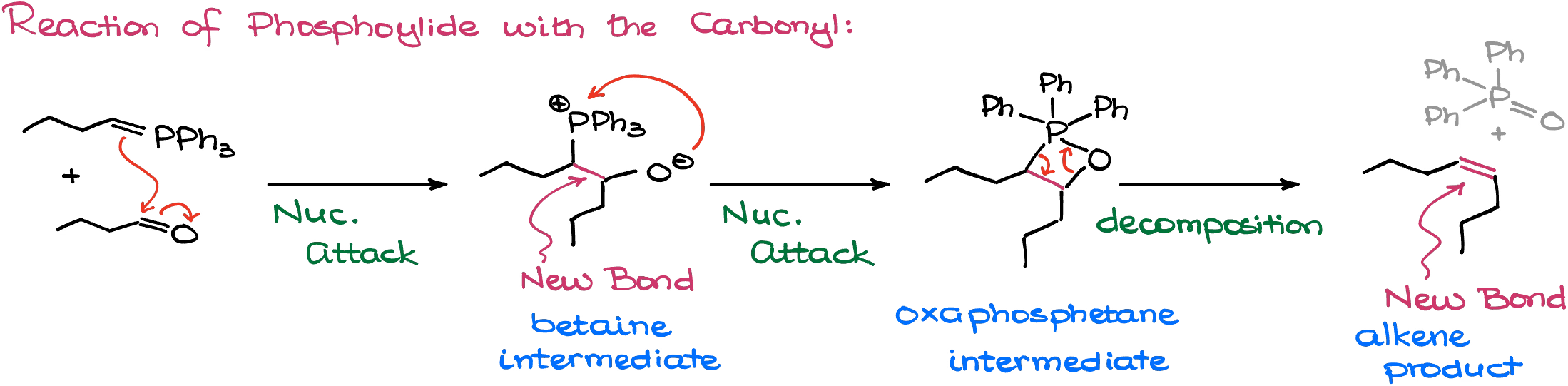
The “classic” mechanism starts with the nucleophilic attack from the carbon of the ylide onto our carbonyl making an intermediate that we call a “betaine.” Essentially, betaine is a zwitterionic compound that can be isolated depending on how exactly we perform this reaction. However, betaines by themselves are quite unstable and tend to quickly cyclize to form the next intermediate called “oxaphosphetane” which is a 4-membered ring with oxygen and phosphorus.
The 4-membered ring, as you can probably imagine, is not that stable either. Interestingly enough, this ring undergoes a spontaneous decomposition forming an alkene and triphenylphosphine oxide. The driving force behind this reaction is the formation of the P=O bond, which is incredible thermodynamically stable.
Alternative Wittig Reaction Mechanism
There’s also another mechanism that you might see in your course. This mechanism bypasses the formation of the betaine intermediate and gives the corresponding oxaphosphetane right the way.
![[2+2] mechanism of the wittig reaction](https://www.organicchemistrytutor.com/wp-content/uploads/2024/03/04-alternative-mechanism-of-the-wittig-reaction.png)
We’ll classify the first step of this reaction as a [2+2] cycloaddition. If you haven’t talked about the pericyclic reactions and cycloadditions in your course yet, don’t worry about it. Just remember the name and you’ll learn about these reactions later.
As I’ve mentioned earlier, betaines are not all that stable and need some sort of stabilizing force to form. Typically, we see the formation of the betaines in the presence of lithium salts that allow for lithium cation to coordinate around oxygen and stabilize it. Without lithium in the system, the reaction seems to be proceeding via the [2+2] mechanism.
In my experience, instructors typically cover the “classic” mechanism with the betaine intermediate. So, if your instructor has told you about the betaine, draw the mechanism with the betaine. If your instructor insists on the [2+2] mechanism, go with that one. At the end of the day, it doesn’t matter how you get to your double bond, the result is always the same. And within the scope of an introductory organic chemistry course, we’re not going to see any examples where the mechanistic differences would become detrimental for the outcome.
Stabilized vs Non-Stabilized Ylides
As I’ve mentioned earlier, ylide structure can significantly affect its properties. We’re going to classify all Wittig ylides into two categories: stabilized and non-stabilized.
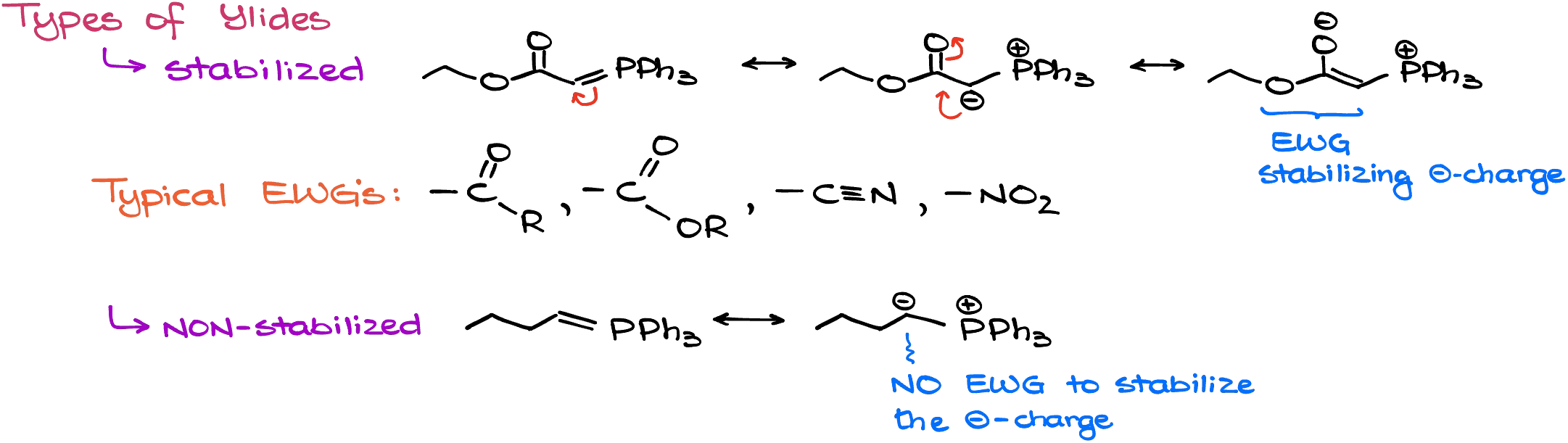
The stabilized ylides have an Electron-Withdrawing Group (EWG) that can stabilize the negative charge on the carbon atom. Typical EWG’s are going to be various carbonyls and other groups that can pull the negative charge towards themselves and stabilize it via resonance. These ylides are typically stable enough so we can easily keep them for the future use.
The non-stabilized ylides, however, do not have an EWG to stabilize the negative charge on the carbon. Because of that, they are very reactive and do not keep well, so we need to use them immediately.
Stereochemistry of the Wittig Reaction
The reason why we pay so much attention to the nature of our ylide is reaction stereochemistry. You see, stabilized ylides tend to give E-alkenes, while non-stabilized ylides tend to give Z-alkenes.
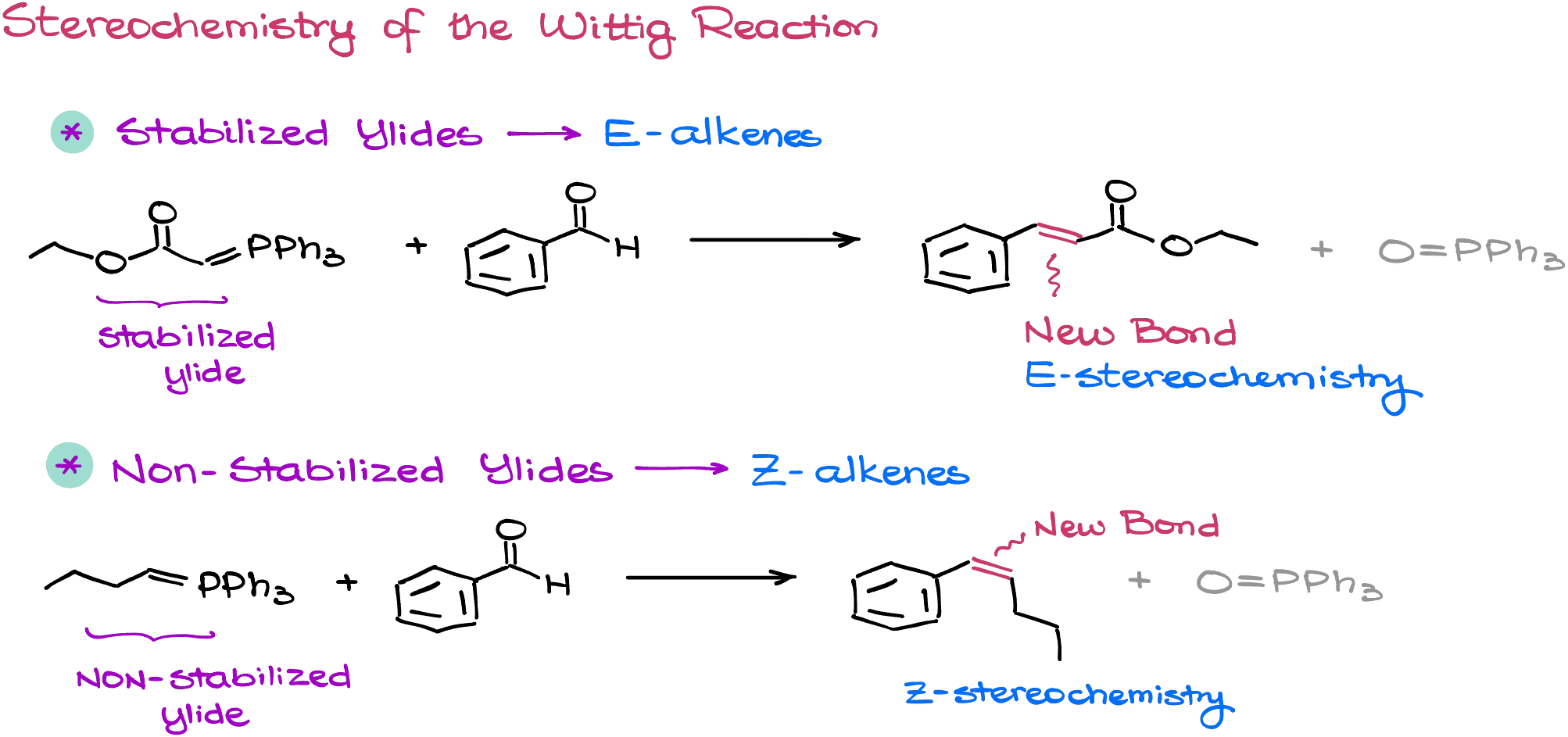
This stereoselectivity is not set in stone and is not absolute either. So, it’s more appropriate to say that we’ll get either E or Z stereoisomer as the major product. And while the stereoselectivity here is not absolute, we typically can expect 80/20 split or better depending on the nature of our reagents.
There are also plenty of modifications of the Wittig reaction that can change this stereoselectivity and the overall outcome of the reaction. However, we typically don’t cover those in an introductory organic chemistry course. So, you’re not likely to see any fancy Wittig versions in your course. But if you do, I’ll talk about those in another tutorial.
