29. Synthesis of Cyclopentanone from Adipic Acid
In this tutorial, I want to talk about the synthesis shown here, where we’re going to start with this hexanedioic acid—also called adipic acid if you prefer the common name—and through several steps, we’ll end up making cyclopentanone.
Since we’re going from an open-chain molecule to a ring, I know that the key step in this synthesis is going to be a cyclization reaction. And because we’re working with carboxylic acids and their derivatives—based on the functional groups in the starting material—the most likely key step here is a Dieckmann condensation reaction. That’s essentially the same thing as a Claisen condensation, except it happens within the same molecule, which makes it intramolecular and perfect for forming rings.
Now, the Dieckmann condensation specifically works with esters, which already gives me a pretty clear idea of what my first step needs to be. I’ll need to convert my carboxylic acids into esters. So I’m going to start with a simple Fischer esterification, using ethanol as the alcohol and tosic acid as my catalyst. That gives me the corresponding diester.
To carry out the Dieckmann condensation, we need to look at one of the α-positions. We’ve got one over here and another one over there, and because the molecule is completely symmetrical, it really doesn’t matter which α-position we choose. Let’s say we number the molecule from 1 to 6 and focus on the α-position at carbon 2. During the condensation, we’re going to form a new carbon-carbon bond between carbon 2 and carbon 6. That gives us a five-membered ring—1, 2, 3, 4, 5—which is exactly what we’re aiming for.
To make this happen, we bring in sodium ethoxide as our base. Then, after an acidic workup to neutralize the intermediate, we get our five-membered ring. If we renumber the atoms from 1 to 6 as in the original molecule, we can see that the new bond formed is between carbons 2 and 6—just as planned. So I’ll go ahead and mark that new bond right there.
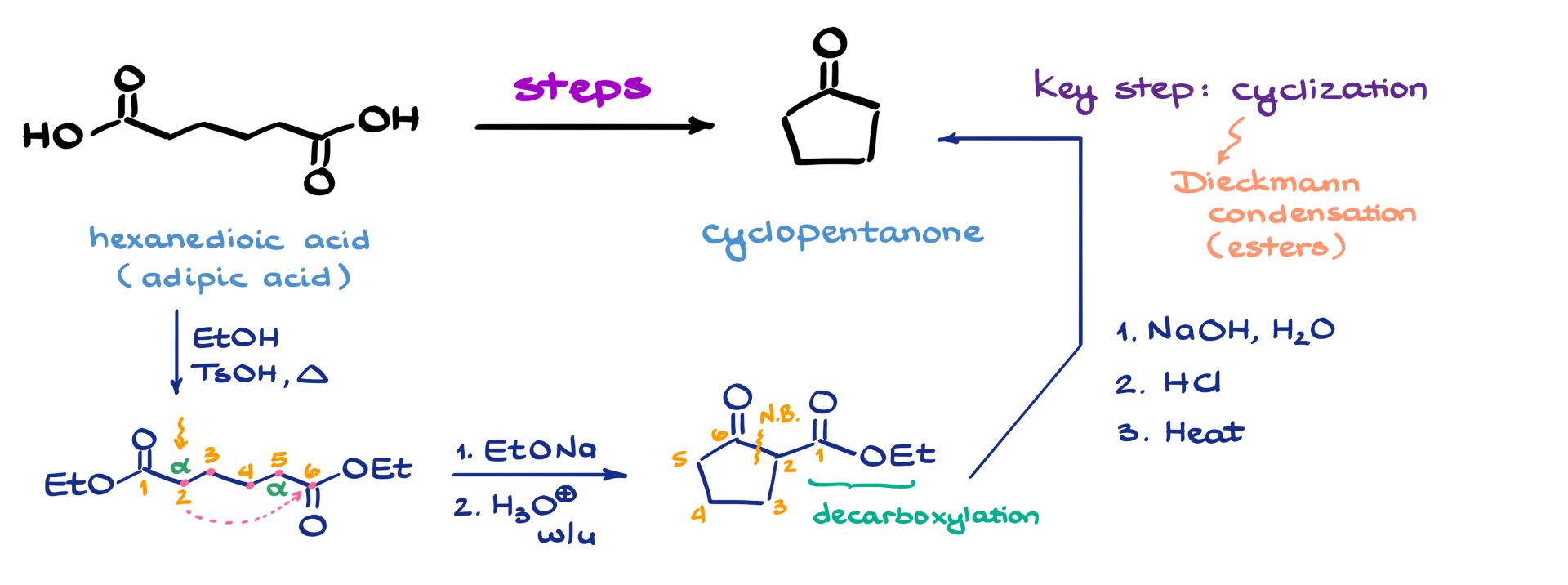
At this point, the only thing left to do is remove carbon 1, which is still hanging out as part of the ester group. To do that, we need a decarboxylation reaction. That’s actually a multi-step process: first, we hydrolyze the ester into the corresponding carboxylate. Then we neutralize it to the carboxylic acid. Finally, we apply a little heat to drive the decarboxylation and generate our final product.
So as you can see, this is a fairly straightforward synthesis—as long as you recognize that the Dieckmann condensation is the key step.
Now, here’s a bit of a challenge for you: how could you modify just one step in this synthesis to end up with an extra methyl group on the final product? Let me know your thoughts in the comments below.
