Synthesis of Aldehydes and Ketones
Synthesis of aldehydes and ketones is a common midpoint in many synthetic sequences that you’re going to see in your course. So, let’s look at the most common reactions that you’ll need to know to ace your tests.
There are four common strategies you’re most likely going to use when you need to make an aldehyde or a ketone:
- Oxidation of primary or secondary alcohols,
- Ozonolysis of alkenes,
- Hydration and hydroboration of alkynes,
- Partial reduction of carboxylic acid derivatives.
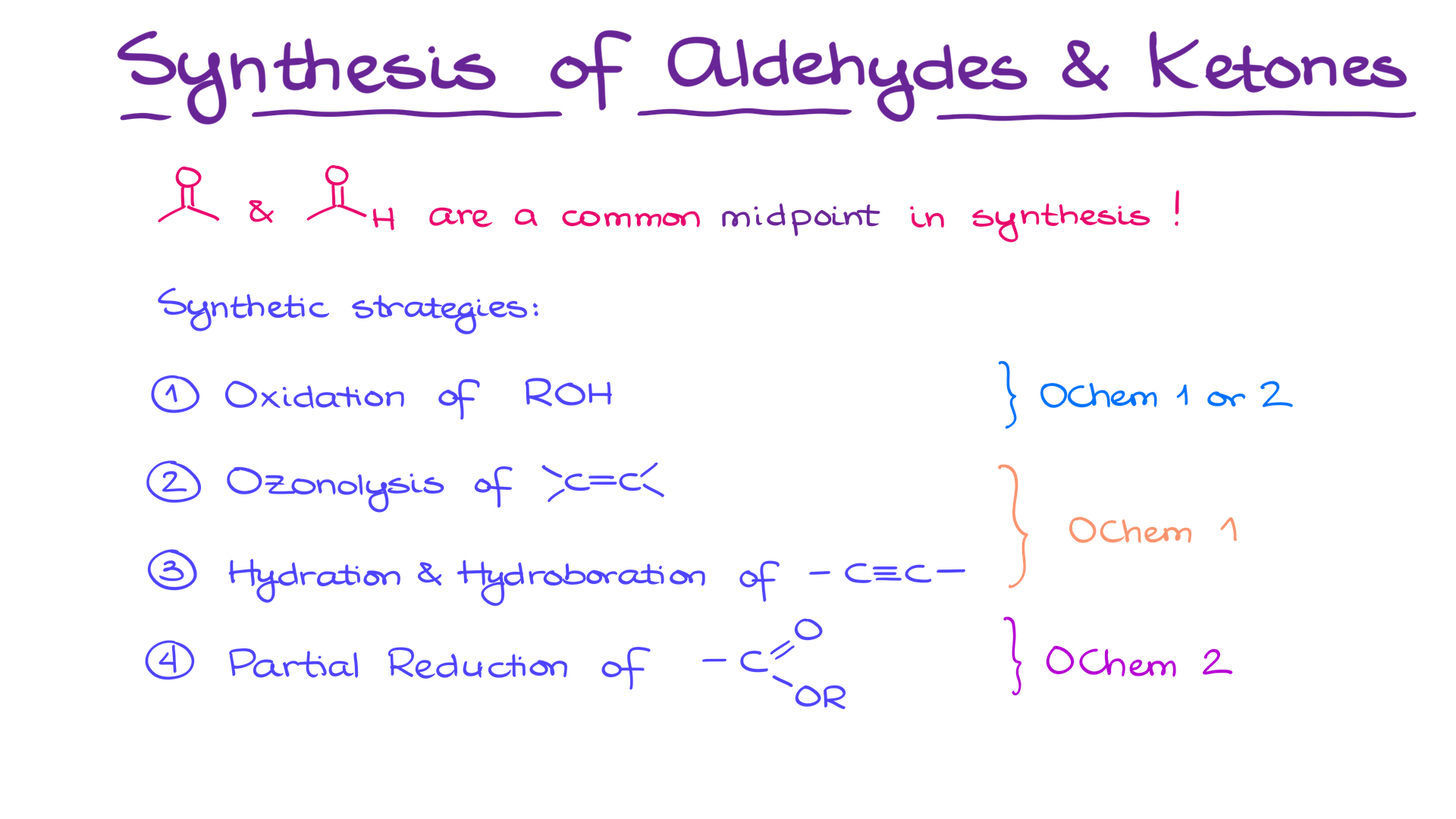
If you’re in the second semester organic chemistry, you’re most likely familiar with the first three methods and will learn the last one later this semester. And if you’re reviewing for your final, well, hopefully you remember them all.
In any case, let’s look at each of these methods and briefly discuss the important highlights of each reaction that you’ll need to know.
Oxidation of Alcohols
Let’s start by looking at probably the most common method — Oxidation of Alcohols. We classify alcohols as 1°, 2°, and 3°. However, only 1° and 2° alcohols can undergo oxidation.
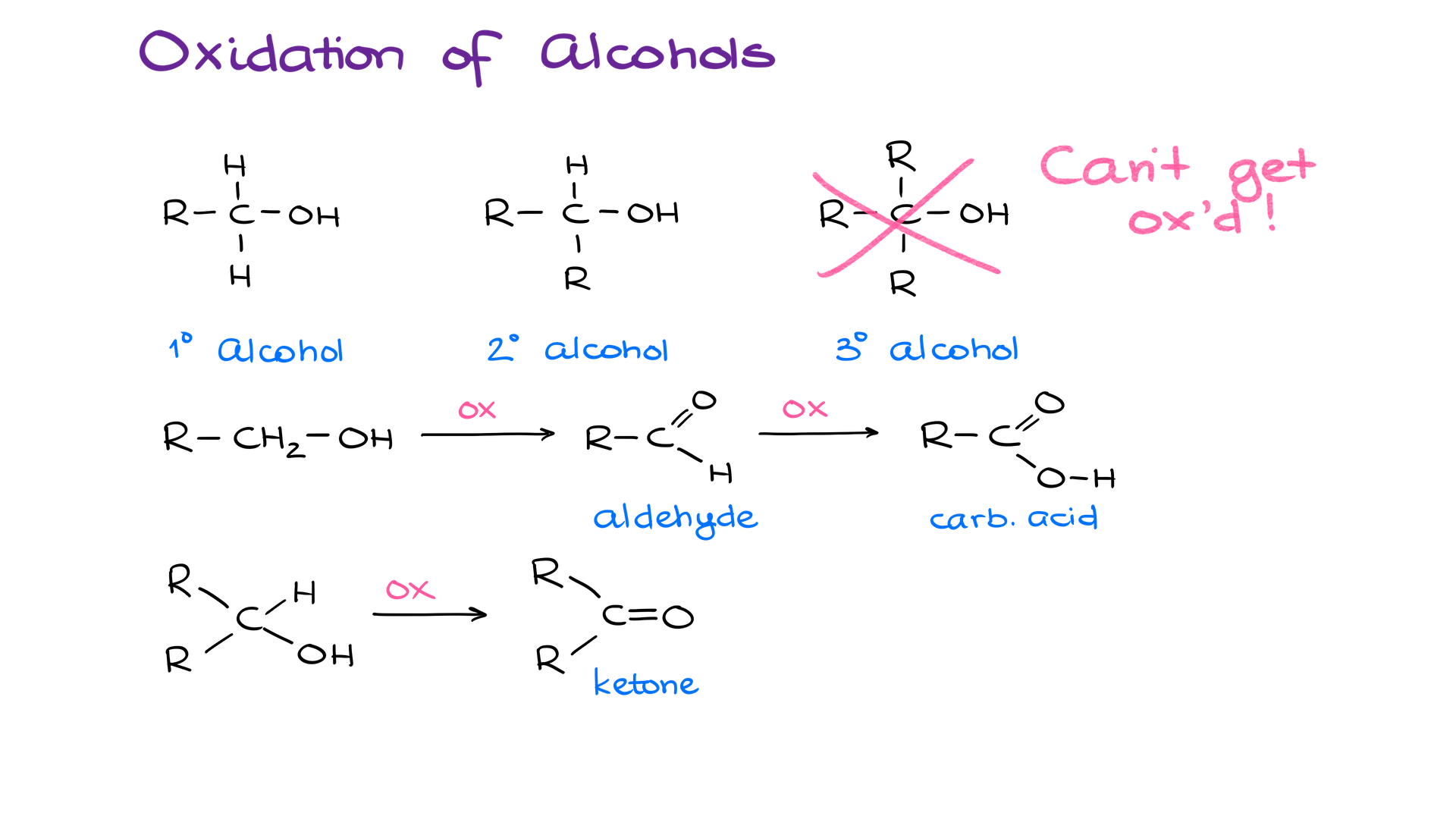
Primary alcohols can make either aldehydes or carboxylic acids depending on the nature of the reagent you’re using and the conditions. Secondary alcohols, however, can only make ketones. So, while it’s irrelevant which oxidizing agent you’re using with secondary alcohols, you gotta be very careful with the primary alcohols.

If we wanna stop at the formation of an aldehyde and not over-oxidize our primary alcohol to carboxylic acid, we’ll need, as I’ve mentioned a moment ago, to use special conditions and reagents. The most common reagents for the partial oxidation of our primary alcohols are PCC, PDC, Swern Oxidation, and Dess-Martin Periodinane. There are a few other methods that you might encounter if you dig a bit deeper into this topic, but these four are the most commonly taught reagents in a sophomore course. For our purposes, they are essentially interchangeable.
Ozonolysis of Alkenes
The next method — ozonolysis of alkenes — is not nearly as popular as a synthetic technique because we typically look at it as a reaction that cuts through the carbon-carbon bonds rather than a reaction that creates a new functional group. Nonetheless, ozonolysis is a perfectly fine method of making an aldehyde or a ketone that we can use when other methods are not available for whatever reason, or when we, well, need to cut some bonds in the process!

Most commonly, you’re going to be using this method when dealing with cyclic alkenes and are needing to cut your ring open while making some carbonyls in the process. Many instructors like to use this trick as a starting point or even a key step in a synthesis on the test. So, I’m willing to bet, you’ll come across this reaction once or twice in your second semester when working on some homework problems or prepping for the test.
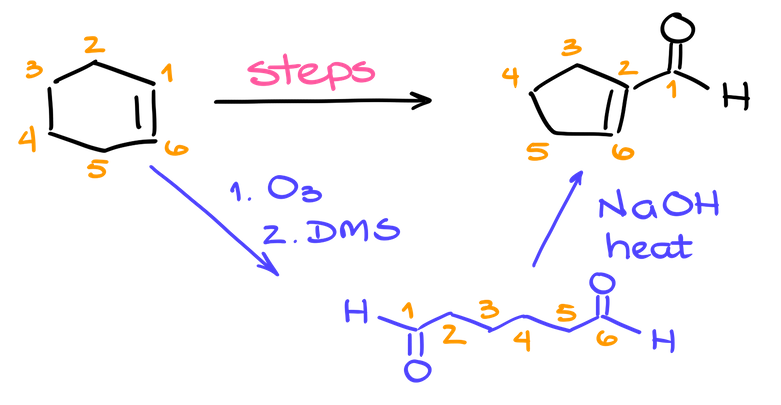
One of my personal favorites that I’ve seen a million times on tests and included it in my tests as well is the synthesis where we start with cyclohexene and then end up with a cyclopentene or some derivative of it. And the key step in this synthesis would be to open our cyclohexene via the ozonolysis reaction followed by the aldol condensation to close the intermediate dialdehyde into a 5-membered ring. Pretty cool, right?
Hydration and Hydroboration of Alkynes
Moving on to the next method, we have hydration and hydroboration of alkynes. Alkynes, as you very well know, are a pretty versatile functional group, and they are relatively easy to make via an alkylation reaction of alkynide ions.
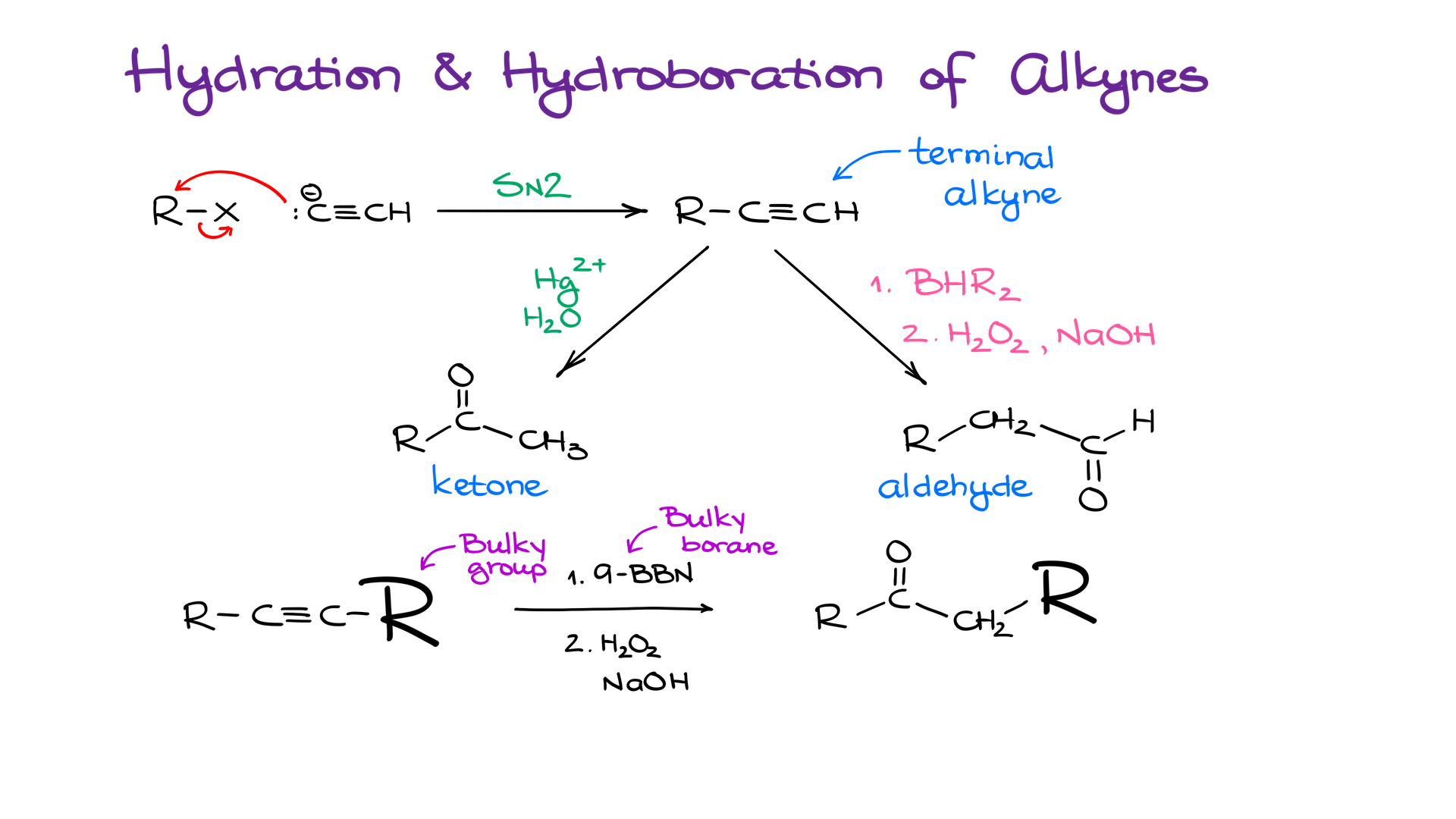
Due to the opposite regioselectivity of the hydration and the hydroboration reactions, we can also easily synthesize either an aldehyde or a ketone in the case of the terminal alkynes. You can also achieve decent regioselectivity with internal alkynes when using bulky boranes and one side of your molecule is significantly larger than the other to cause sufficient steric interferences.
And again, while this method is not as universal and straightforward as oxidation of alcohols, it can be useful in some situations. So, it is a good idea to keep this method in your synthetic toolkit.
Partial Reduction of Carboxylic Acid Derivatives
And finally, we have one last method you should know — partial reduction of carboxylic acid derivatives. Typically, what I see most students do when they need to convert a carboxylic acid or its derivative into a corresponding aldehyde, they completely reduce it to a corresponding primary alcohol with something like lithium aluminum hydride and then oxidize the resulting alcohol into the aldehyde. While this is a fine sequence and it gets the job done, it’s far from efficient.
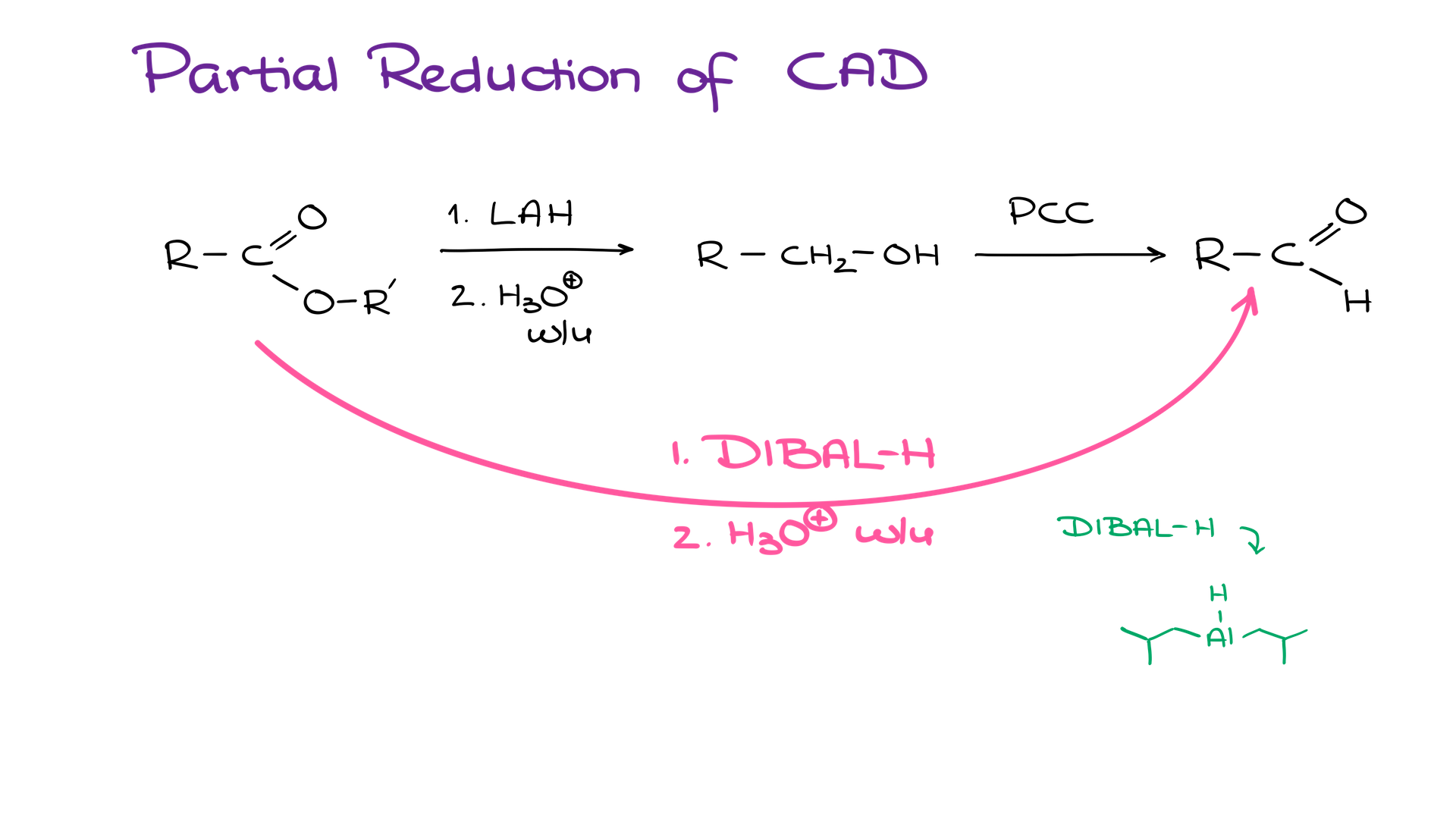
There’s a way you could do it directly by using reagents like DIBAL-H (di-isobutylaluminum hydride) at a low temperature. This reaction works for esters and acid chlorides and affords you an aldehyde in one synthetic step.
There are a few versions of this reaction and a few modifications of the reducing agent that you can use here. So, if you’re curious, you might wanna look into this reaction a little closer. However, within the scope of a typical sophomore organic chemistry course, we usually limit ourselves to DIBAL-H.
Concluding Thoughts
So, to wrap it up, when you’re going to be looking at making an aldehyde or a ketone, you’re most likely going to be making it from the corresponding alcohol. You could also use alkenes, alkynes, and CAD’s to make your aldehydes and sometimes ketones as well.
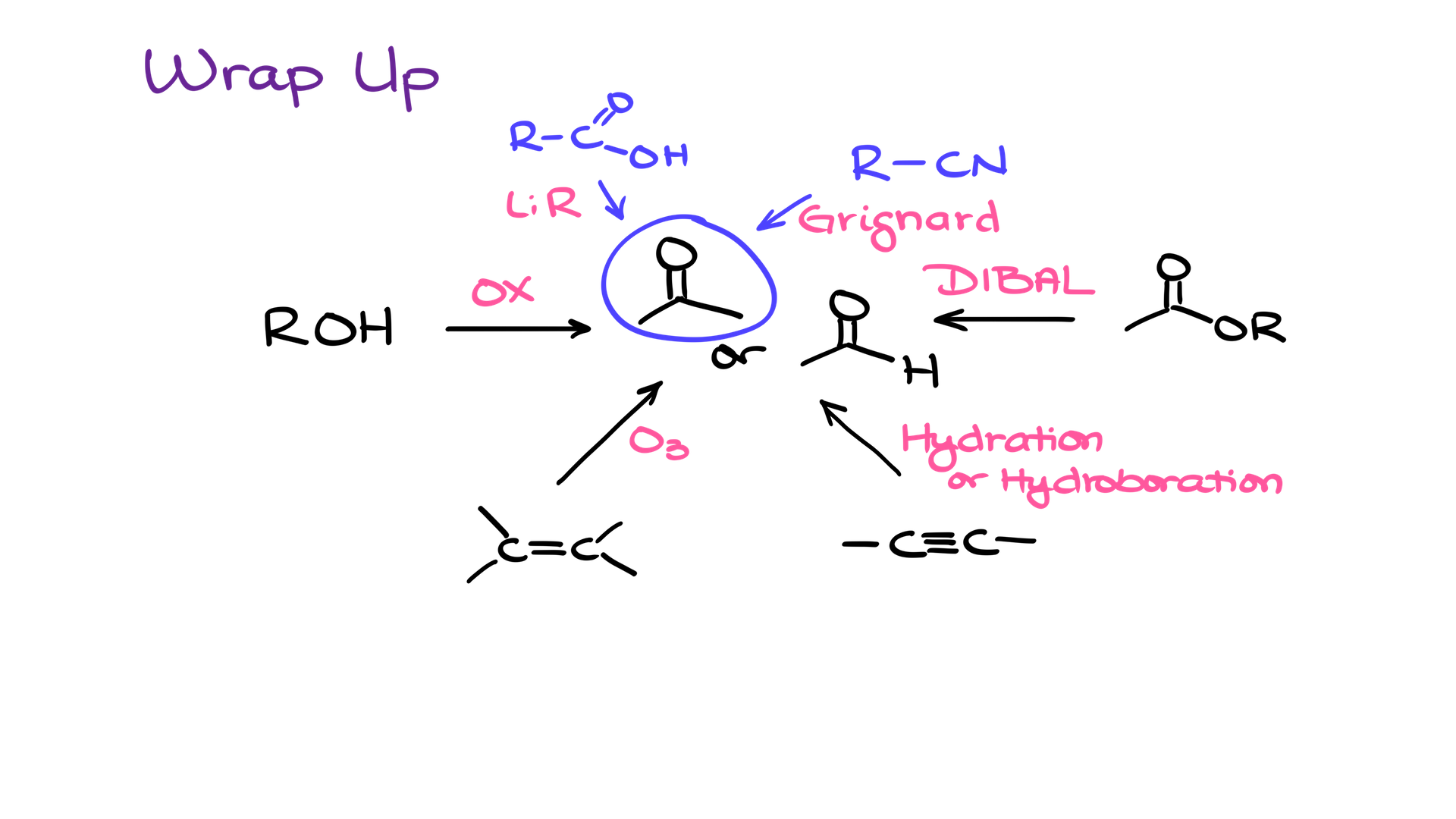
This list, of course, is by no means exhaustive! There are other methods that afford carbonyls. Specifically, you can make ketones via a Grignard reaction with nitriles or by reacting organolithium compounds with carboxylic acids. Those reactions will kill two birds with the same stone by not only making a carbonyl, but also creating a new carbon-carbon bond.
