30. Synthesis of a Complex Ketone from an Ester
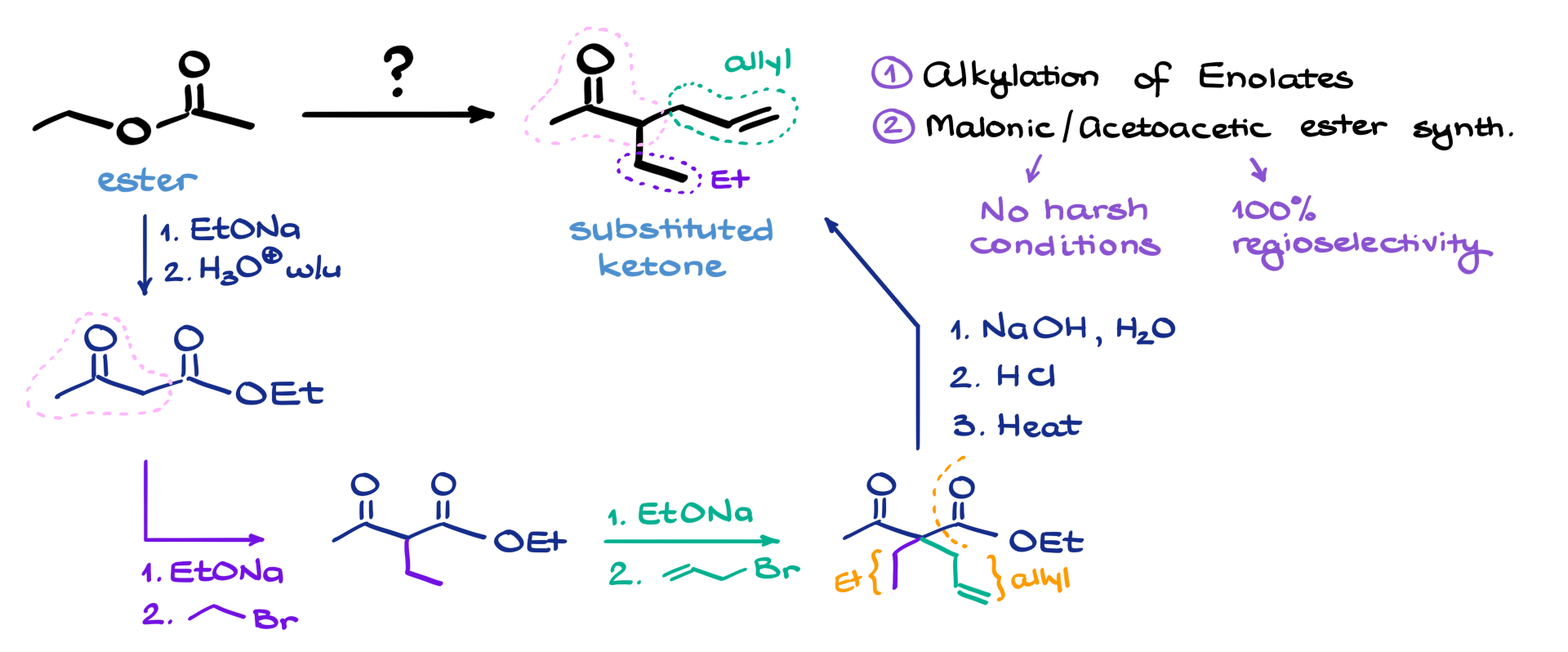
In this tutorial, I want to talk about this fun synthesis. We’re starting with an ester, and after several steps, we’re going to end up with this substituted ketone over here.

So, since we’re starting with a carbonyl compound in this case and we’re going to be forming a couple of new bonds—one over here and another one here—it’s most likely that we’re looking at either alkylation of enolates or possibly a malonic or acetoacetic ester synthesis, which is basically another version of enolate alkylation, just with a twist.
Personally, I prefer using the malonic or acetoacetic ester synthesis every time, and that’s for two main reasons. First, I don’t have to use harsh reagents like strong bases—no need for LDA, sodium hydride, or anything like that. And second, and even more importantly, these reactions always give 100% regioselectivity, which is super important in any synthesis.
Now, you might be thinking: “Wait a second, our starting material is just ethyl acetate, not a malonic or acetoacetic ester.” And you’re absolutely right. But here’s the thing—if I take ethyl acetate and subject it to a simple Claisen condensation with itself, I’ll end up with acetoacetic ester. So while I don’t have it right at the start, it’s just a one-step process to make it.
From that point on, I can see how the acetoacetic ester maps onto the structure of our final product. That tells me that I need to add an ethyl group and an allyl group to my molecule, and then finish it off with a decarboxylation reaction.
Now, the exact order in which I add those groups doesn’t really matter, so I’ll start with the ethyl group. To do that, I’ll deprotonate my acetoacetic ester and treat it with ethyl bromide, which will give me the product with the ethyl group attached.
Then, to add the allyl group, I’ll use the same approach: deprotonate the molecule again, treat it with allyl bromide, and I’ll get the desired product with both groups now attached. So here’s my ethyl group right here, and the allyl group over here—I’ll just label it like that.
The only thing left is to get rid of the ester group on this part of the molecule. We can easily do that through decarboxylation. First, I’ll hydrolyze the ester under basic conditions, then neutralize the mixture to get a β-ketocarboxylic acid, and finally heat it up a bit to drive off CO₂ and finish with the final product.
Pretty easy!
