Stobbe Condensation
In this tutorial, I want to look at this rather interesting reaction. At first glance, it might seem like a simple aldol condensation, where we’re just forming an alpha-beta unsaturated compound like this. But if we look closer—what’s the deal with this carboxylate over here, and why is the other ester untouched? Well, that’s because this isn’t just a simple aldol condensation. This reaction is called Stobbe condensation, and it has a rather interesting mechanism. So let’s take a look at it.

Mechanism of the Stobbe Condensation
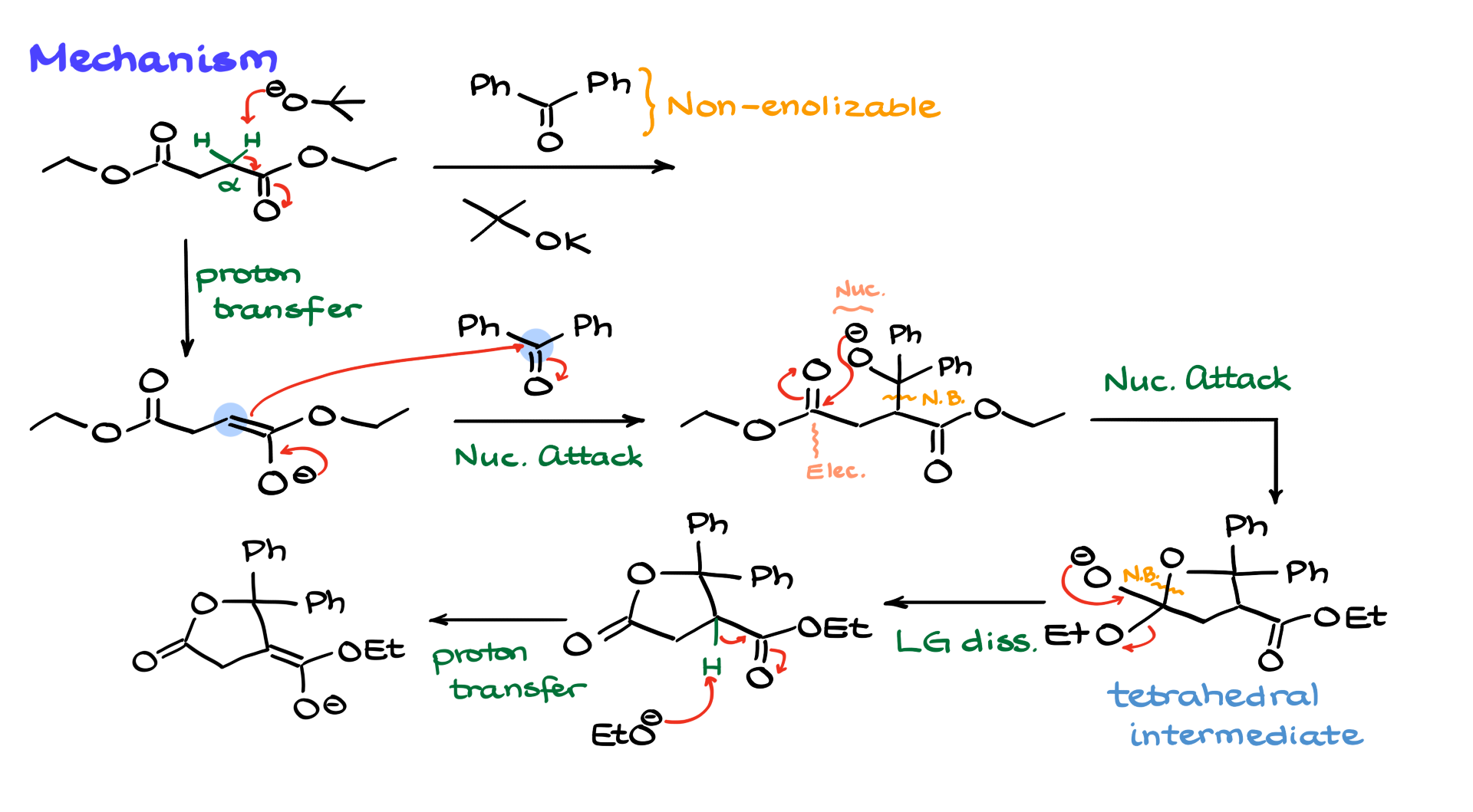
Here are my starting materials and reagents. I have this diester as my starting material, I have my ketone—benzophenone—and I’m also working with a base. The first thing that jumps out at me right away is that we have a non-enolizable ketone, which means the only enolizable position available is the alpha position to my ester. And since I have a symmetrical diester, it doesn’t matter which alpha position I choose. So, I’ll go with this one.
Now, I’m going to show the alpha protons and the base. What’s going to happen here is that the base will come in and pull one of those protons off, forming our enolate. The next step is to bring in our carbonyl. Since the carbonyl is going to act as the electrophile, and the enolate is our nucleophile, the enolate will react with the electrophilic carbonyl like this, forming a new carbon-carbon bond between the alpha position of our enolate and our electrophile—our carbonyl—giving us the following negatively charged intermediate. And, of course, the new bond we just created is right here.
Now, in a normal aldol condensation, the next step would be protonation of the O⁻. However, in this case, the O⁻ is actually going to act as a nucleophile. Since this is a negatively charged species, it’s nucleophilic, and interestingly, right next to that nucleophile, we have an electrophile in the form of the carbonyl. So instead of a proton transfer, our nucleophile and electrophile will react with each other, forming a new carbon-oxygen bond right here. That gives us a normal tetrahedral intermediate, and as with any tetrahedral intermediate, it will kick out a leaving group. In this case, the leaving group is ethoxide, and we get the following five-membered ring as the product of this step.
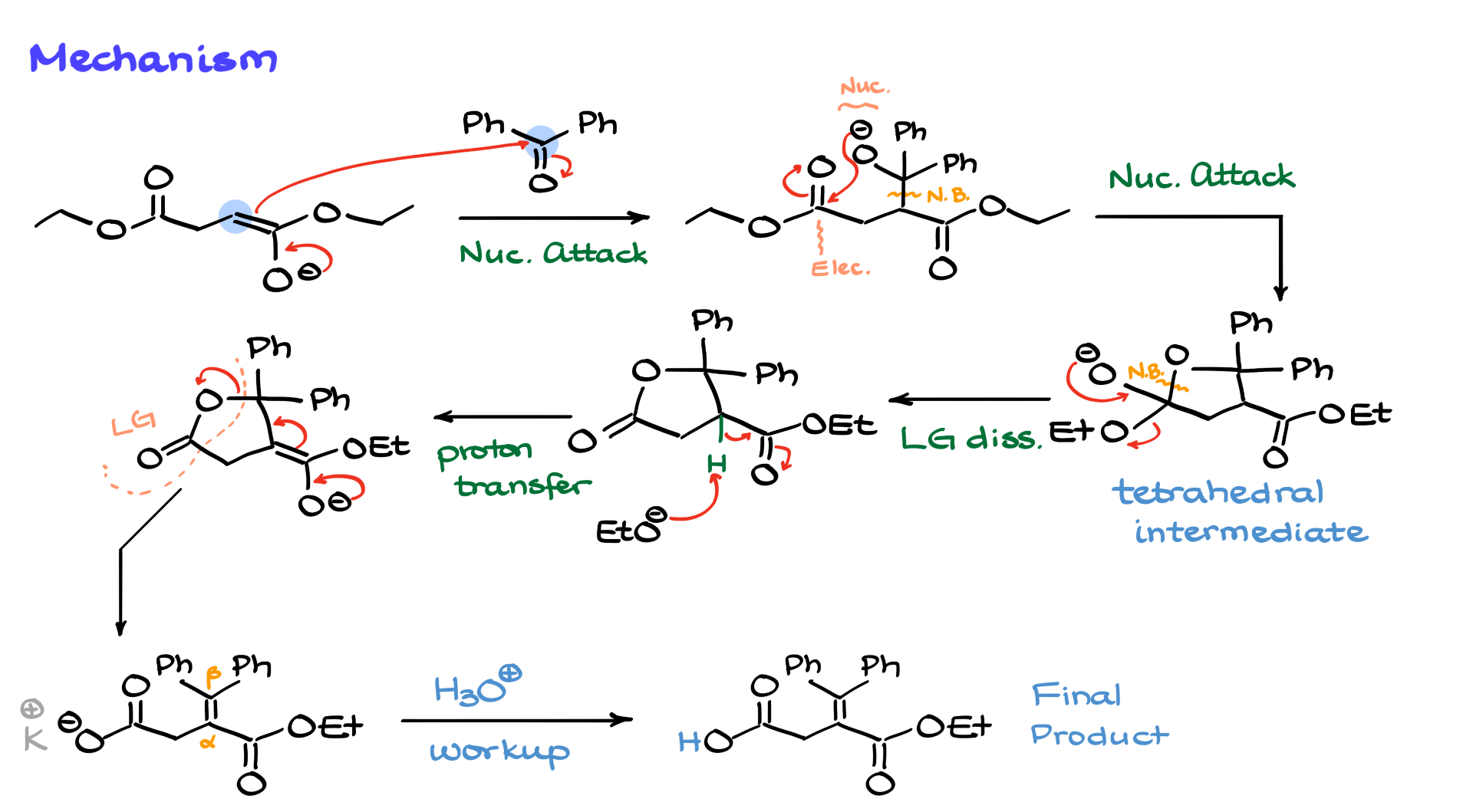
But here’s the deal—we just kicked the ethoxide out, but we still have an enolizable position next to the carbonyl. That means the base will come in again and re-enolize that position, giving us the following enolate intermediate. Let’s clear some space here—cool.
Now that we have this enolate, the next thing it can do is kick out our leaving group, which in this case is essentially this portion of the molecule. That breaks the carbon-oxygen bond and creates our alpha-beta unsaturated compound over here. At this point, if we don’t want our compound to remain negatively charged, we can simply note that the counterion here is potassium. And if we don’t like that, we can do an acidic workup to obtain the final product, which in this case is a neutral carboxylic acid.
So, this is how we end up with a molecule where one ester is hydrolyzed while the other remains unchanged. Pretty cool, huh? Have you ever seen a mechanism like that before?
