SN2 Reactions
In this tutorial we are going to talk about the SN2 reactions which is, probably, the easiest of the four classic mechanisms that we are going to cover in this unit. So, first, let’s talk about what the “SN2” abbreviation means.
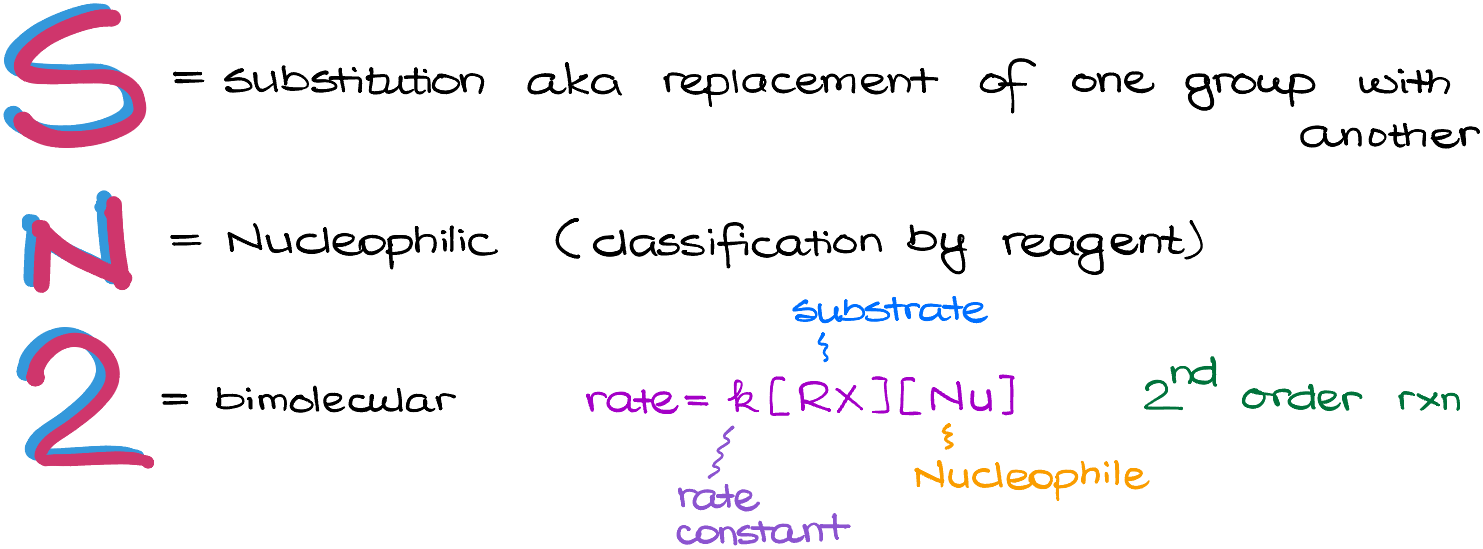
S— stands for Substitution, meaning that this is a reaction in which we are going to replace one group in the molecule with something different.
N—stands for Nucleophilic . Here, we are using so-called classification by the reagent. Our molecule, that we call a substrate, is going to be reacting with a nucleophile. Thus, this is a nucleophilic reaction. For instance, if we look at the reaction in the following example, we have 1-bromobutane, which is our substrate, reacting with the sulfur containing nucleophile.

Sodium in this reaction is just a spectator ion, so we can pretty much ignore it. Now, if we look at the product of this reaction, we will see add that we end up with a molecule in which bromine (which is our Leaving Group) is replaced with the nucleophile.
Finally, the number 2 stands for the bimolecular reaction. This means that we are dealing with the second order reaction in which the reaction rate depends on the concentration of both: the substrate and the nucleophile.
For the reaction in this example we can write the rate of this reaction as the expression rate=k[BuBr][CN–].
In this expression, we have our substrate and our nucleophile included in square brackets representing their concentrations. And as we already mentioned earlier, the sodium cation is merely a spectator ion and is irrelevant for our reaction, so we’re not even going to include it in here.
Bimolecular Substitution is a Concerted Mechanism
Here is another example of an SN2 reaction.

In this reaction, the Leaving Group (I–) is replaced with the nucleophile (CN–). And like in the previous case, spectator ion (K+) is irrelevant, so we can cross it out. From the mechanistic perspective, this reaction is an example of a concerted mechanism. This means that the nucleophilic attack happens at the same time as the Leaving Group dissociation. You can think about this process like if we have a cylinder with two pistons. As we insert one piston into our cylinder, it will push out the other piston that is already in the cylinder from the other end. So, no intermediates of any sort are formed in this reaction.
Energy Diagram of an SN2 Reaction
A concerted mechanism means the absence of the intermediates. This also means that the energy diagram for the SN2 reaction will look like this:
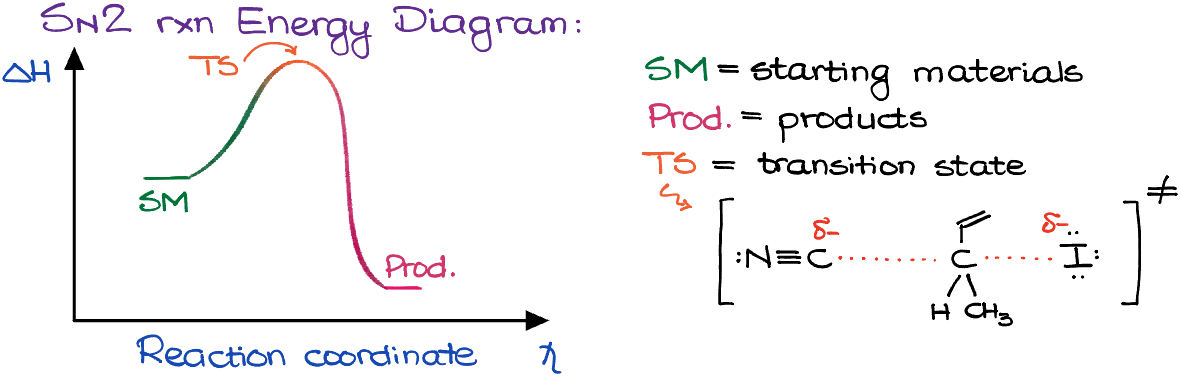
I also want to point out that the transition state is the molecular entity which resembles both starting materials and products and exists no longer than a single vibration. So, the transition state is not something that we can isolate. Rather, it is something in between your starting material and products. In our energy diagram, the transition state is represented by the top of our curve.
Stereochemistry of the SN2 Reactions
The nature of the transition state and this reaction brings me to the next important aspect of the SN2 reactions—the stereochemistry of it. The SN2 reaction occurs with the strict inversion of the stereochemistry because we have to have the backside attack from our nucleophile onto our electrophile. Essentially, if our Leaving Group is on the dash, like in my example here, the nucleophile has to attack from the opposite face of the molecule, and because of that it will end up on the wedge. Likewise, if my Leaving Group was on the wedge, the nucleophile would be on the dash in the final product. Like in the following example:
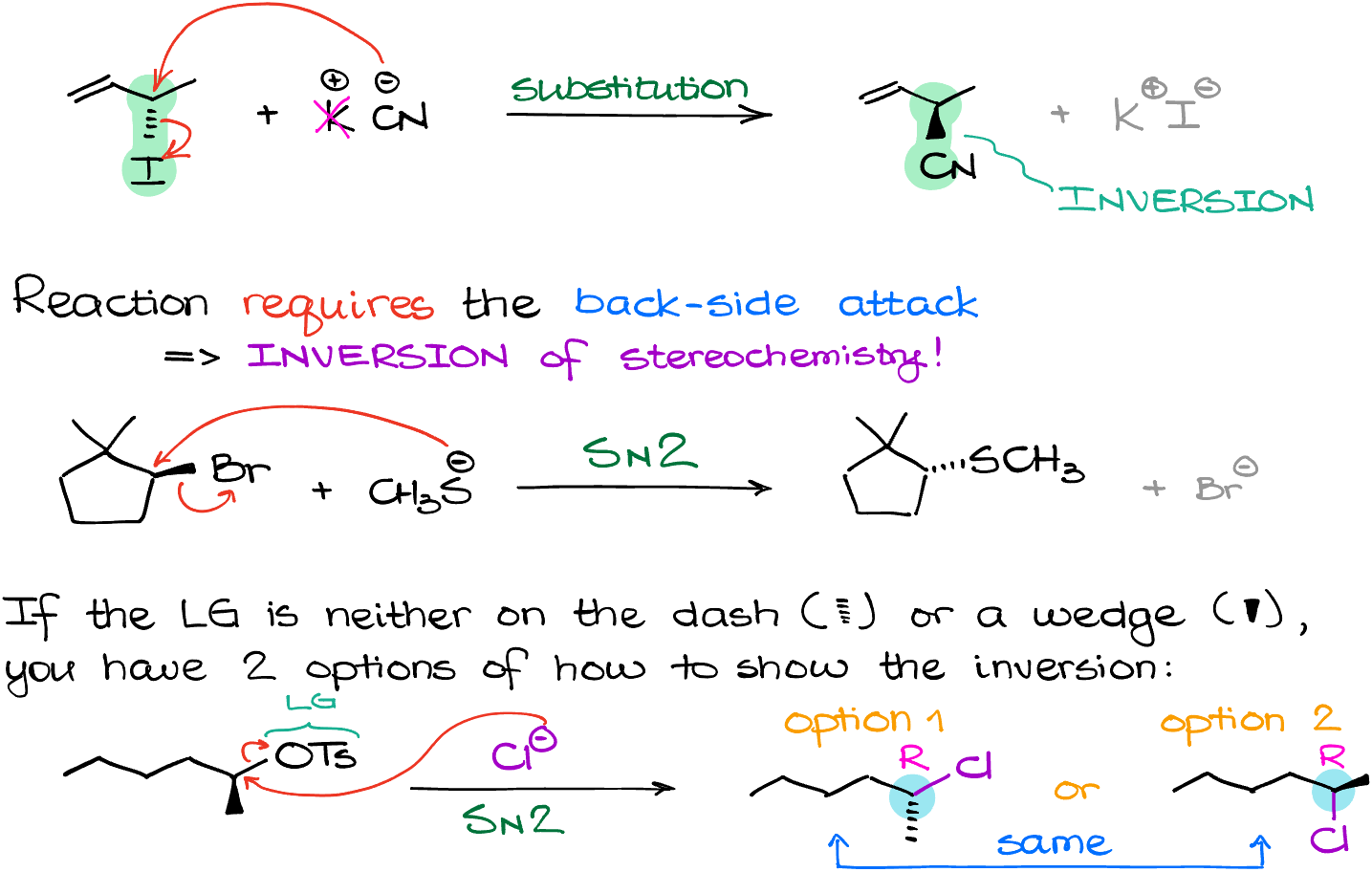
But what if my Leaving Group is neither on the dash or the wedge? What then? Well, we have two options then.
Option 1: just flip the stereochemistry of whatever group you have on the wedge or dash.
…or alternatively…
Option 2: you can “flip” your stereocenter like an umbrella in a high wind.
Here is something important that I wanna point out while we were talking about the inversion of the stereochemistry in our molecule. Let’s look at this example:
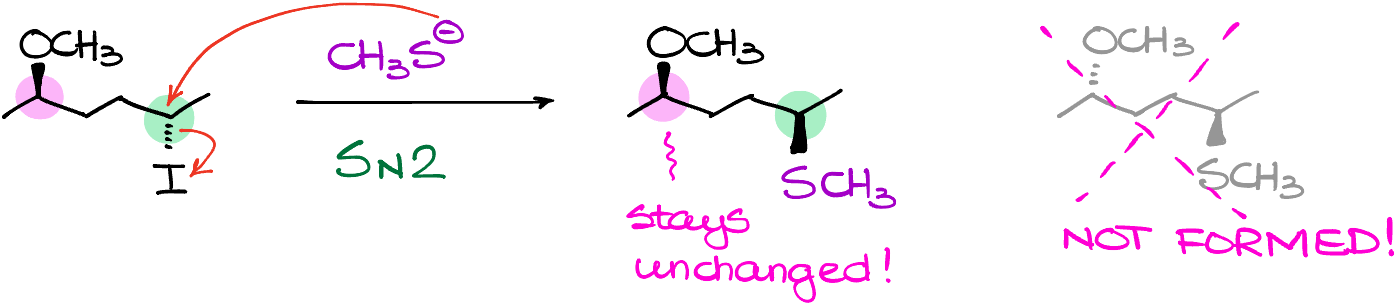
We have two chiral atoms in this molecule, I will highlight them with green and pink. Since our reaction only occurs at the green atom, the pink one will stay unchanged. Be very careful and pay close attention to where you are doing the actual reaction and don’t blindly flip everything in your molecule. This is a very common trick on the exams, and you can certainly expect that your instructor will try to catch you on that. Also, if the atom with the leaving group is not chiral there is nothing to flip. So, pay attention to that as well. Here is one of my favorite trick questions:
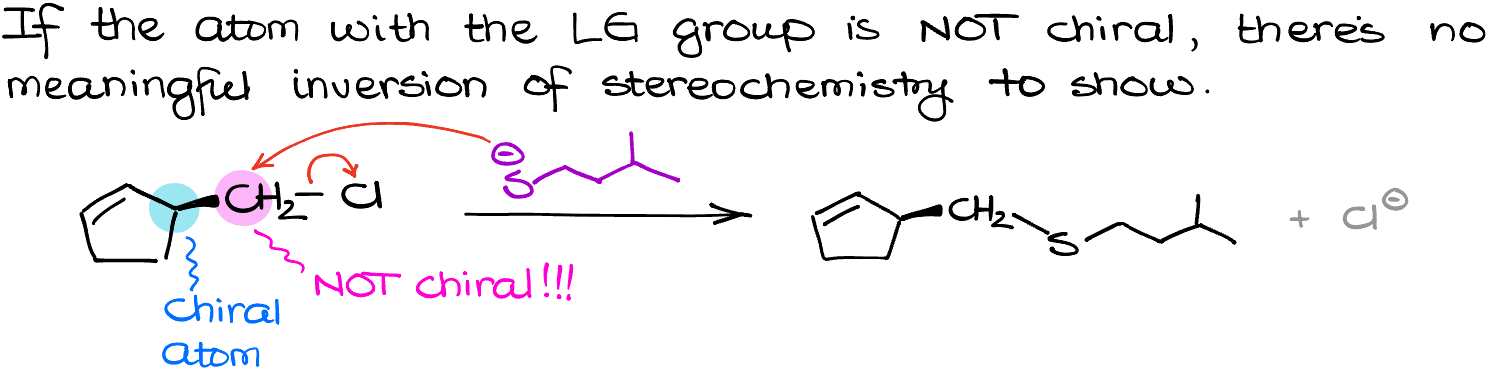
As you can see, we do have a chiral atom in this molecule.
BUT
The chiral atom is adjacent to the carbon with the Leaving Group and therefore it stays as is. This gives me the product that looks like what we have in the figure above. So, always be vigilant and pay attention to what you are doing as questions like this are fairly common on exams and I can guarantee that your instructor will try to catch you it was one of those.
Alright, hopefully we’re clear about the stereochemistry inversion in the SN2 reactions. But why do we have the backside attack requirement to begin with? Well, to answer that we’ll have to look at the molecular orbitals.
The σ-bonding orbital and the σ*-anti-bonding orbitals are positioned that 180° to each other. So, if we want to break the bonding orbital and displace our Leaving Group, we need to supply the electron density to the anti-bonding orbital which is at 180° to the bonding one. In other words, the nucleophile simply has no other choice as to attack from the backside of our molecule. Also, since leaving groups of nucleophiles are typically electron-rich species, there will be electrostatic repulsion between them. Thus, if we try to attack our molecule from the same side where our Leaving Group is, the electrostatic repulsion will make such an attack extremely unfavorable.
Steric Hindrances and Reactivity of Substrates in the SN2 Reactions
Also, because of the back-side attack requirement, the SN2 reactions are extremely sensitive towards the the steric hindrances around the electrophilic atom. For instance, let’s look at this lineup of molecules:
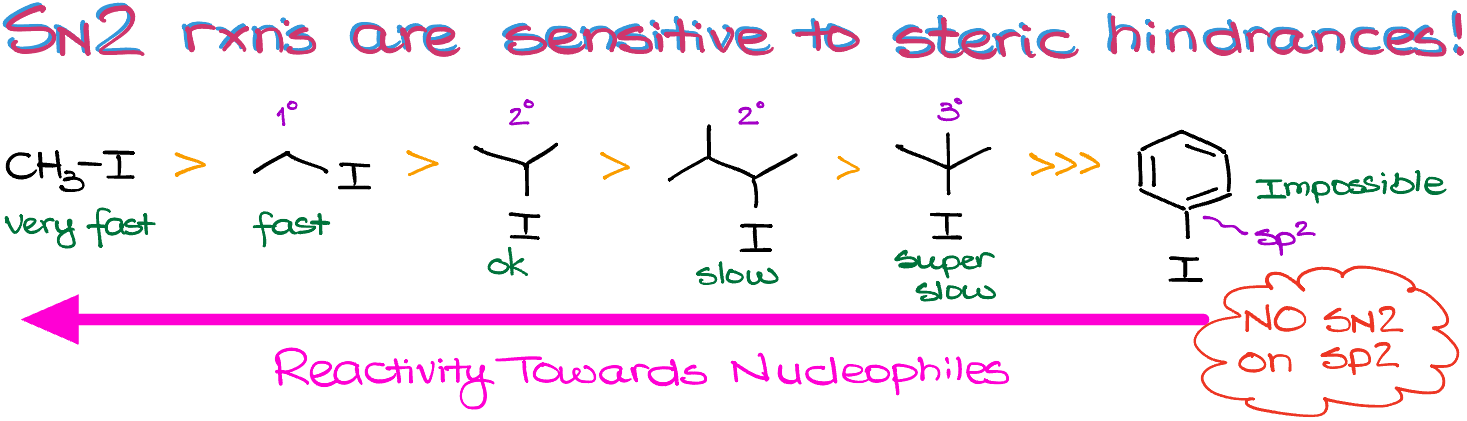
Here, the reactivity towards nucleophiles decreases as we increase the number of substituents on the carbon with the Leaving Group or near it. This makes the CH3-group most reactive, and gradually decreasing the reactivity as we move along.
It is, however, impossible to do the SN2 substitution on an sp2-hybridized atom like in the case of a benzene ring or a simple double bond. I challenge you to build a molecular model here and figure out why that is. Pay close attention to the positioning and the angles around the molecular orbitals. Or you can also remember a simple mnemonic—NO SN2 on sp2!
Polar Aprotic Solvents for SN2 Reactions
Another thing that can help the SN2 reactions is the nature of our solvent. While the solvent is not the determining factor in the mechanism, it facilitates the reaction. The SN2 reactions are favored in the presence of polar aprotic solvents. Here are some of the most common polar aprotic solvents you’re likely to encounter in your course. This list is far from exhaustive, so I suggest you make a cheat-sheet with those and pay attention to which solvents your instructor likes to use in their examples.

Ok, let’s recap what we have just learned.
- SN2 is a bimolecular reaction whose rate depends on the concentrations of both the substrate and the nucleophile.
- SN2 reaction occurs via a concerted mechanism, meaning that all happens in one step and no intermediates are formed.
- Reaction proceeds with the inversion of stereochemistry due to the backside attack of the nucleophile on our substrate.
- Due to the strict requirement of the backside attack, the nucleophilic attack is very sensitive towards the steric hindrances.
- The reaction works best with good nucleophiles. You can always refresh your memory of what a good nucleophile is and what is an electrophile here.
- Finally, the SN2 reactions are facilitated by the polar aprotic solvents.
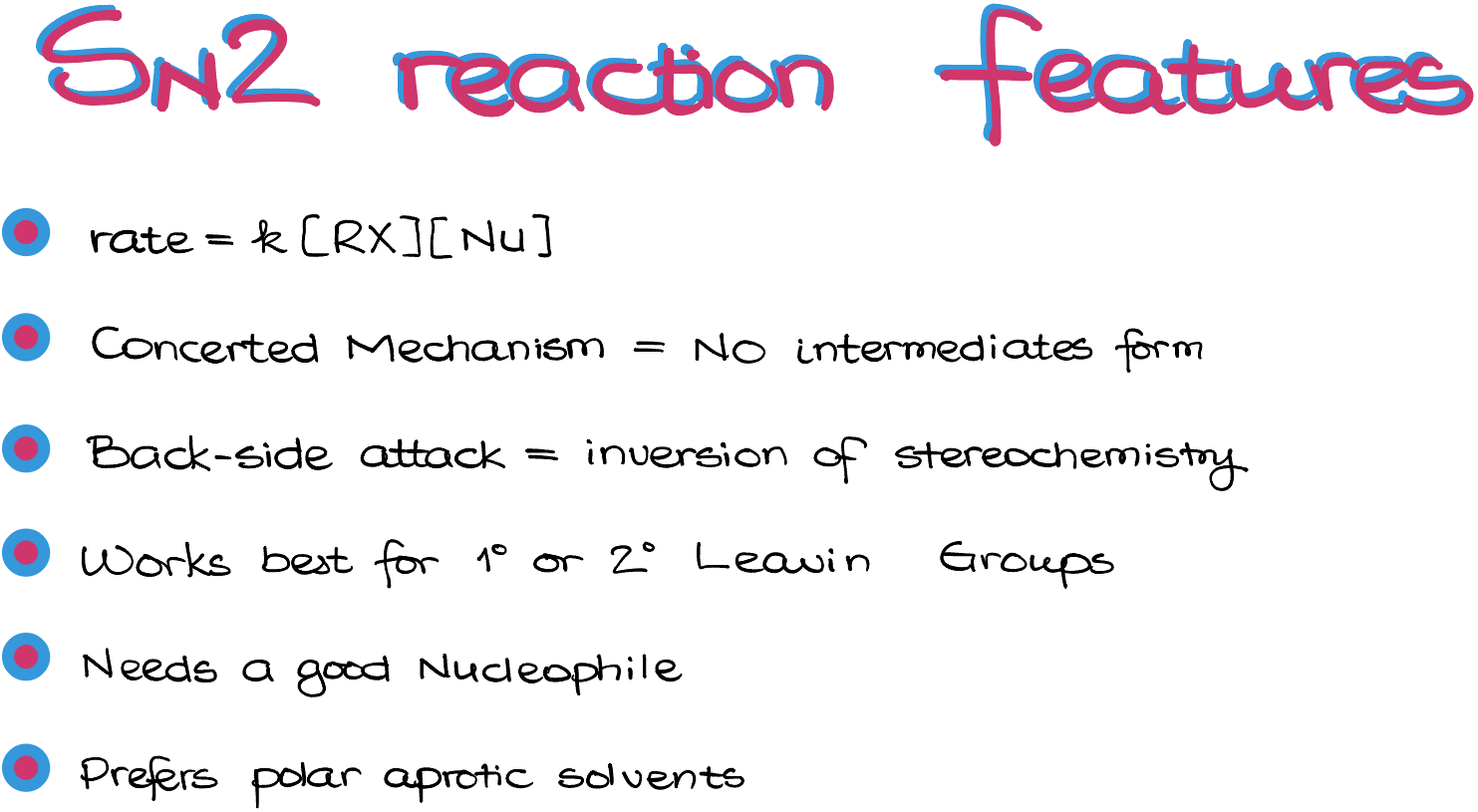

HI Victor
In SN2 reactions can you please explain when to place the leaving group in a wedge position or Dash position?
Thank you
The structure and the stereochemistry of your substrate needs to be given to you, we don’t just decide where to put the leaving group.
Hey Victor, I’ve got a question about the energy diagrams. In your video you’re showing the products being at lower energy than the reagents. Are SN2 reactions always exothermic?
Nope, it really depends on the reaction itself and the bonds you’re making/breaking there.