Sigma and Pi Bonds in Organic Molecules
In this tutorial, we’ll look at the sigma and pi bonds in organic molecules. We’ll begin by reviewing the difference between ionic and covalent bonds, then look closer at how covalent bonds—specifically sigma and pi bonds—form through orbital overlap. Using simple examples like methane, ethylene, and acetylene, we’ll examine single, double, and triple bonds, and discuss how to count these bonds in complex molecules. By the end, you’ll have a clear understanding of the role of hybridization in bond formation and how to identify sigma and pi bonds in organic structures.
Types of Chemical Bonds
First of all, a quick reminder about the two main types of bonds we see in molecules.
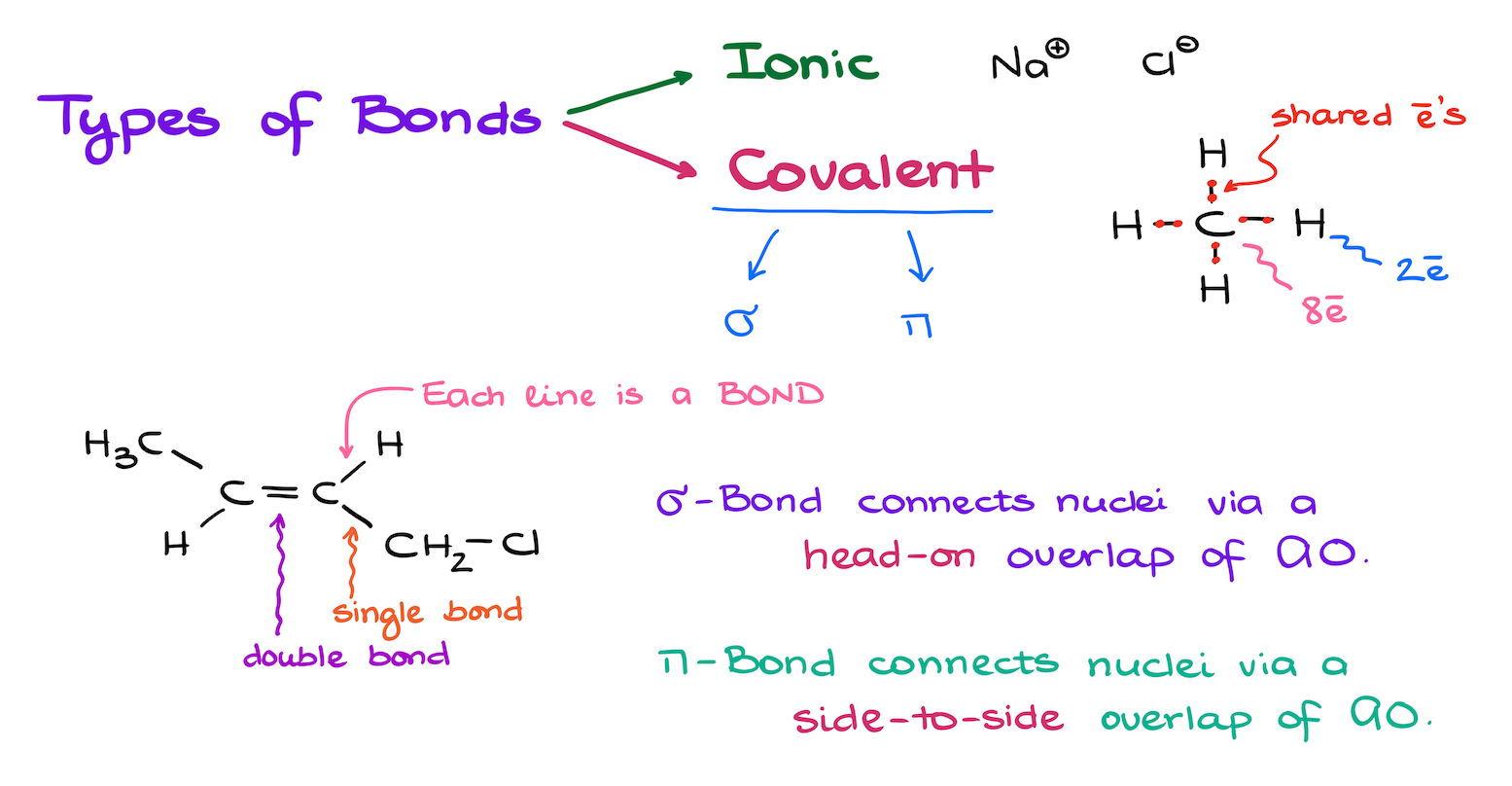
The first type is the ionic bond, in which one element completely gives away its electrons. For instance, sodium chloride is a good example of an ionic compound where the sodium atom gives its valence electrons to the chlorine atom. As a result, sodium has a positive charge, and chlorine has a negative charge because it has gained extra electrons.
While we do see some ionic bonding in organic molecules, we don’t typically focus on those types of bonds. They’re more common in inorganic compounds. What’s more interesting to us is the covalent bond.
In a molecule built with covalent bonds, the electrons aren’t given away but rather shared between atoms. For example, the pair of electrons between carbon and hydrogen are shared. Each hydrogen ends up with two electrons around it as a result of this sharing while each carbon has eight.
However, the ionic vs. covalent bond distinction isn’t the only thing we need to know about chemical bonding. We further break down the covalent bonds into the sigma and pi bonds.
Sigma Bonds
Let’s start by looking at how sigma bonds form. In order to make an H₂ molecule, we need two hydrogen atoms. When these atoms come together, their electron clouds overlap. This overlap occurs along the line that directly connects the nuclei of the two atoms, a process known as “head-on overlap.” This is an example of a sigma (s) bond.
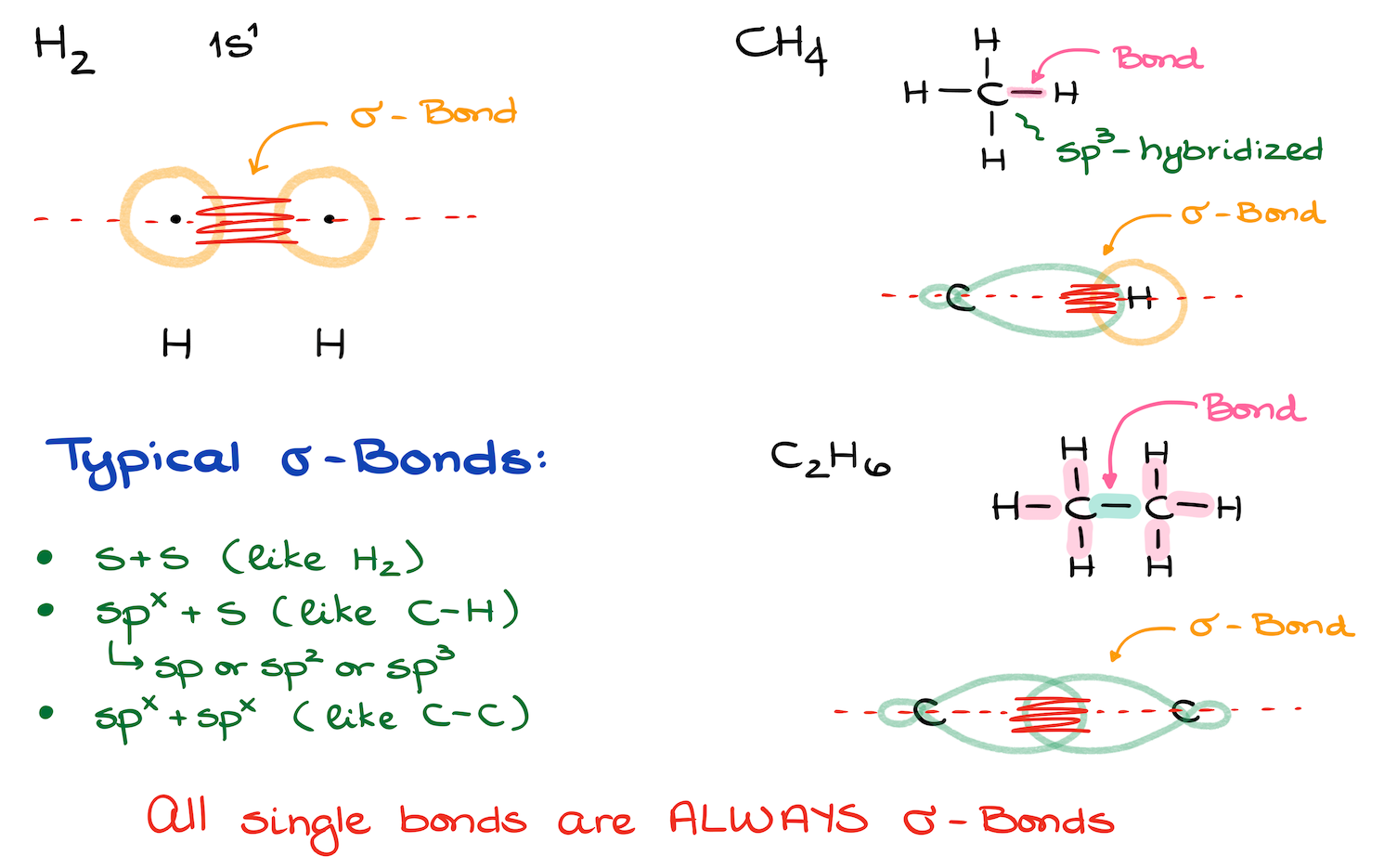
Now, let’s take a more complex example. Every single line in a structural drawing represents a bond. So, if we look at the bond between carbon and hydrogen under magnification, we’ll see something interesting.
In methane, the carbon atom is sp³ hybridized, meaning it has four identical sp³ hybrid orbitals. Let’s focus on just one of these sp³ orbitals, which consists of a small lobe and a much larger lobe. Next to this carbon atom is a hydrogen atom, which has its own 1s orbital.
The overlap of these orbitals occurs along the line that directly connects the nuclei of carbon and hydrogen. In this case, the sigma bond forms by overlapping a hybrid orbital from carbon with an s orbital from hydrogen.
Now, when we look at a carbon-carbon bond, such as the one in ethane, we see that both carbons are sp³ hybridized. Just like before, the orbitals overlap directly between the nuclei, forming a sigma bond.
So, to summarize: your typical sigma bonds form either from the overlap of a hybrid orbital (sp, sp², or sp³) with an s orbital, or from the overlap of two hybrid orbitals. Remember, all single bonds are sigma bonds.
Pi Bonds
Things get more interesting when we look at multiple bonds. Let’s take ethylene as an example. Its Lewis structure shows four carbon-hydrogen bonds, which are all sigma bonds. But what about the double bond between the carbons?
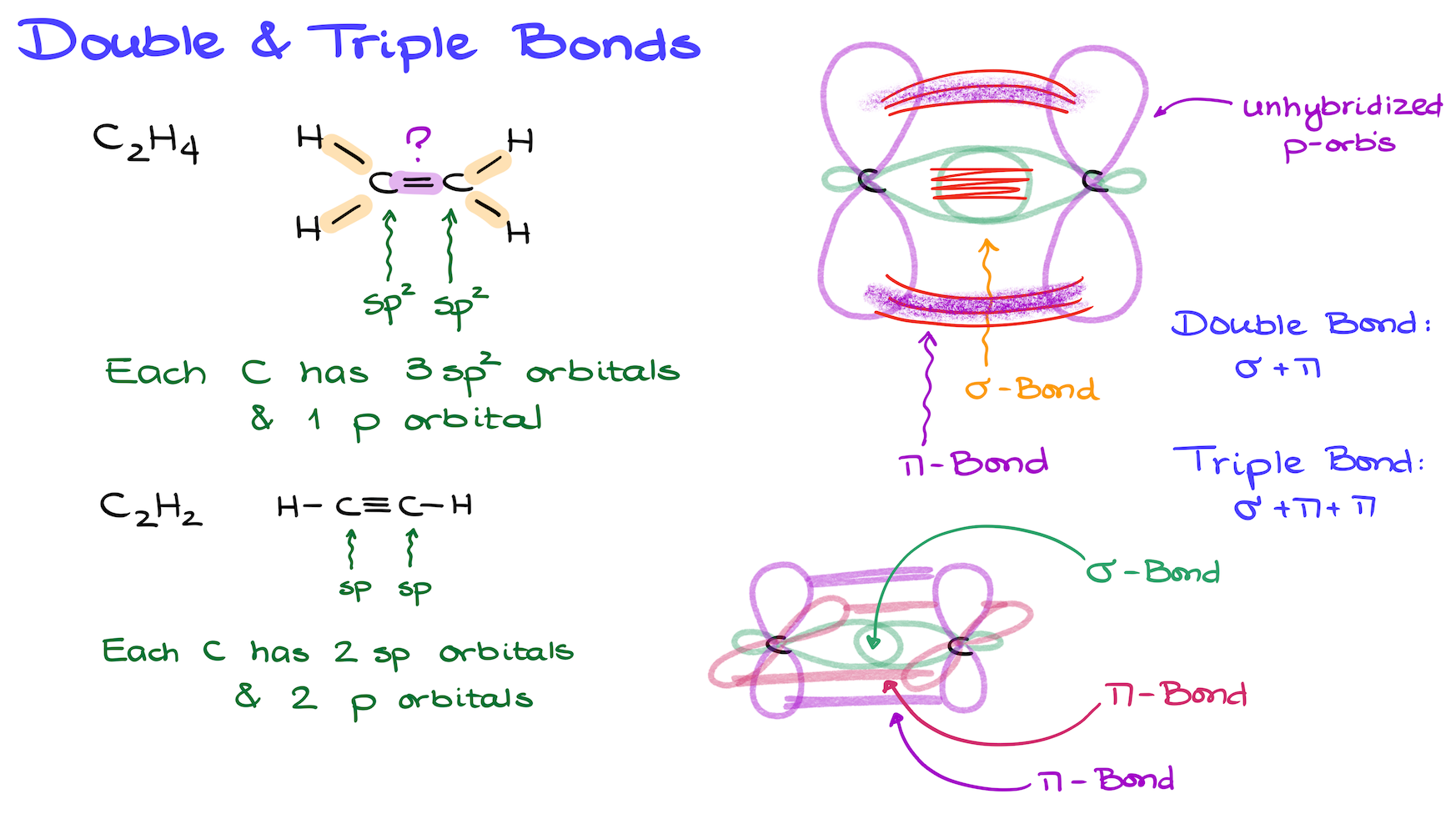
In ethylene, each carbon is sp² hybridized, which means it has three sp² hybrid orbitals and one unhybridized p orbital. If you need a refresher on hybridization, check out this tutorial here.
Now, let’s draw our orbital overlap to see how the bonding works. Each carbon has a p orbital that sticks out perpendicular to the plane of the molecule. These p orbitals overlap, creating a bond above and below the plane of the carbon atoms. This is the pi (p) bond.
A common misconception is that there are two separate bonds when students see two areas of overlap. But no, the entire overlap above and below the carbon atoms is just one pi bond. In this case, the pi bond forms a “cloud” around the sigma bond, which lies directly between the carbons. So, the sigma bond is essentially sandwiched inside the pi bond.
Now, let’s talk about triple bonds, such as in acetylene. Acetylene consists of two carbons, each sp hybridized. Like before, we have a sigma bond formed from the overlap of sp orbitals, but now we have two pi bonds from the overlapping p orbitals.
To summarize: a double bond consists of one sigma bond and one pi bond, while a triple bond consists of one sigma bond and two pi bonds.
Common Exam Question
A common exam question you might encounter is to calculate the number of sigma and pi bonds in a molecule. For example, let’s take the structure of salicylic acid. To do this, I recommend redrawing the molecule to show all the bonds explicitly, especially if you’re still getting comfortable with bond-line structures.
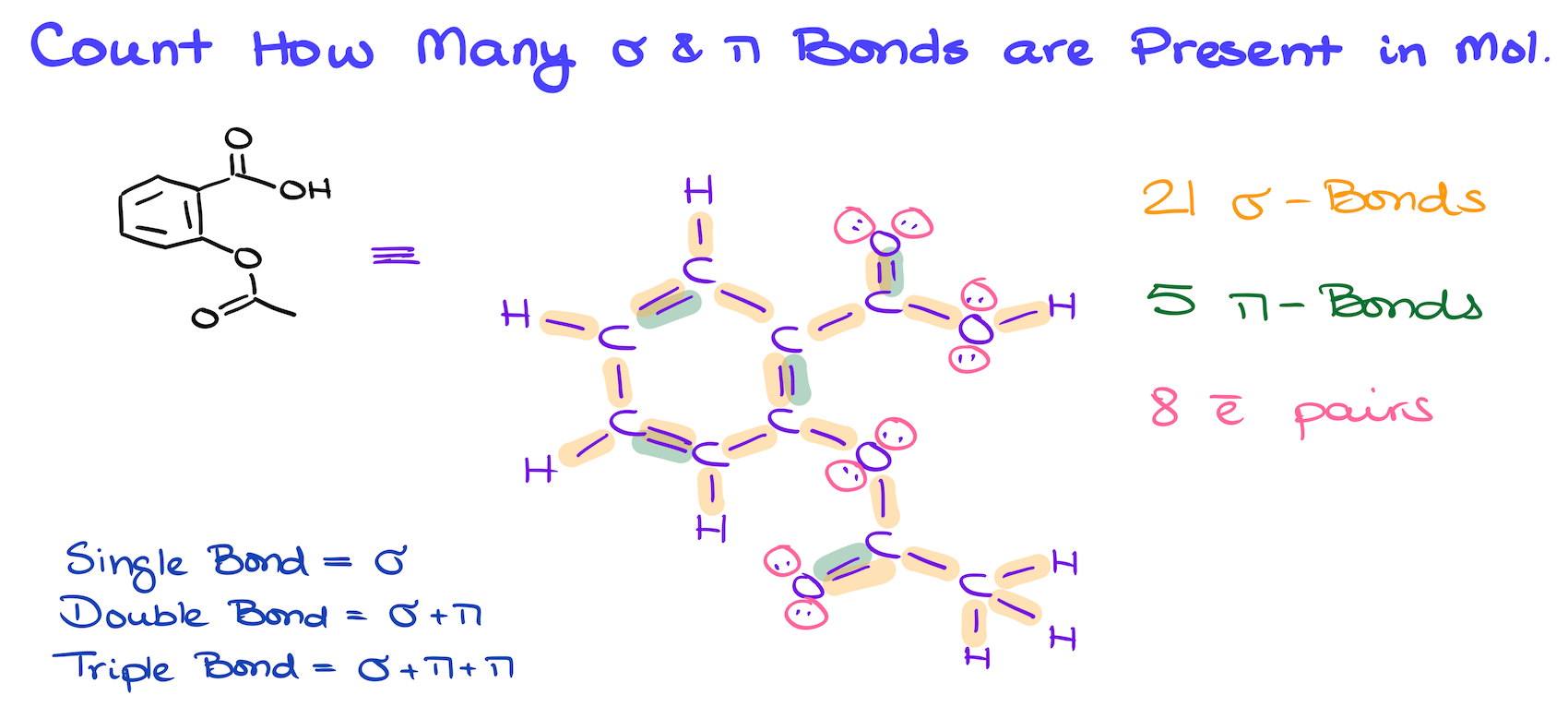
In this molecule, we have 16 single bonds, which are all sigma bonds. We also have a few double bonds, each consisting of one sigma bond and one pi bond giving us a total of 21 sigma bonds, and 5 pi bonds. Finally, don’t forget about the lone pairs of electrons, which are often asked about in these kinds of questions.
So, remember, single bonds are sigma bonds, double bonds are one sigma plus one pi bond, and triple bonds are one sigma plus two pi bonds. Easy enough, right?
Practice Questions
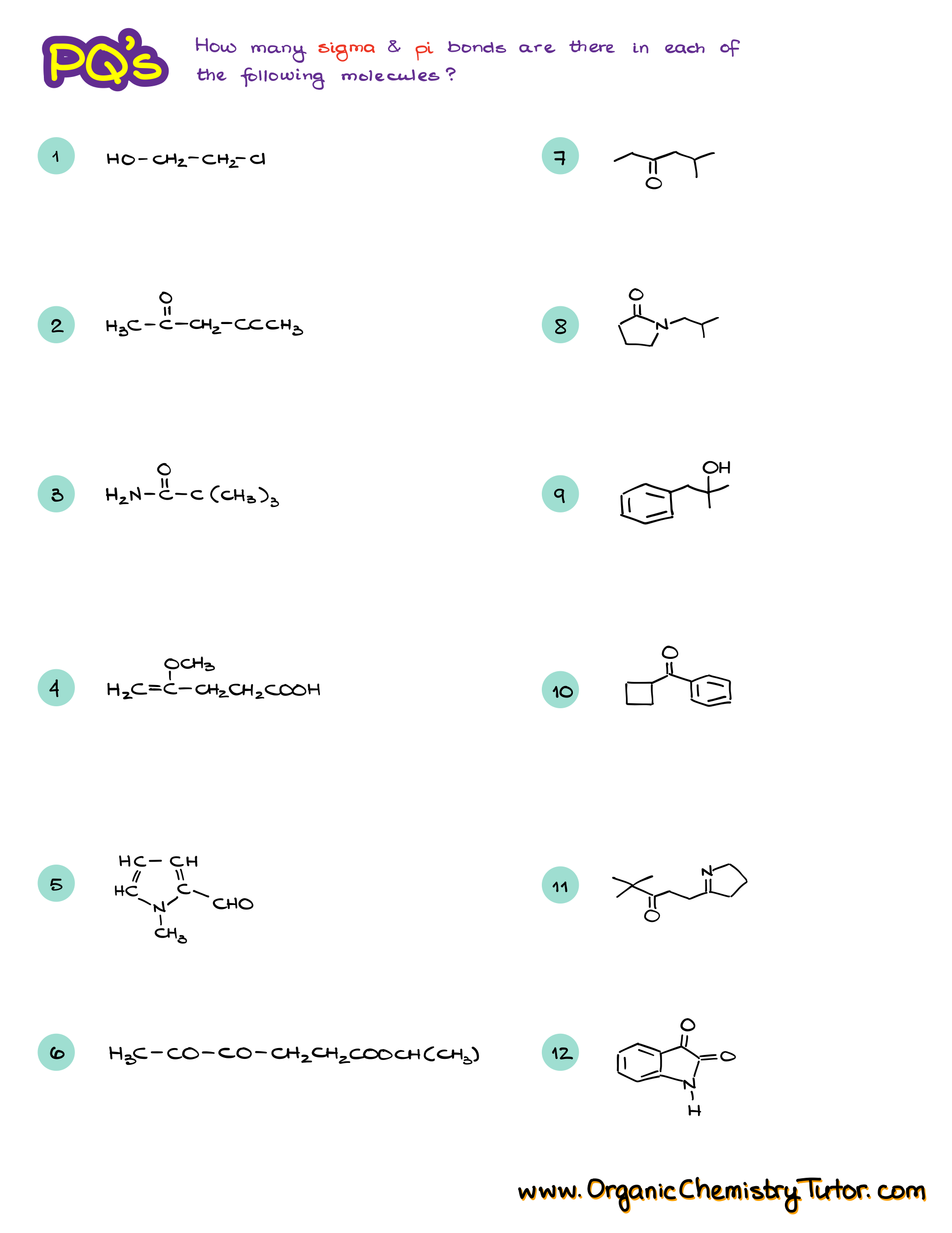
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!
