Saponification of Esters
In this tutorial we’re going to take a look at the saponification reaction, which is a type of acyl substitution reaction. This process takes an ester, a derivative of a carboxylic acid, and reacts it with a base—typically sodium or potassium hydroxide. The reaction yields two key products: a carboxylate, which is the salt of a carboxylic acid, and an alcohol.
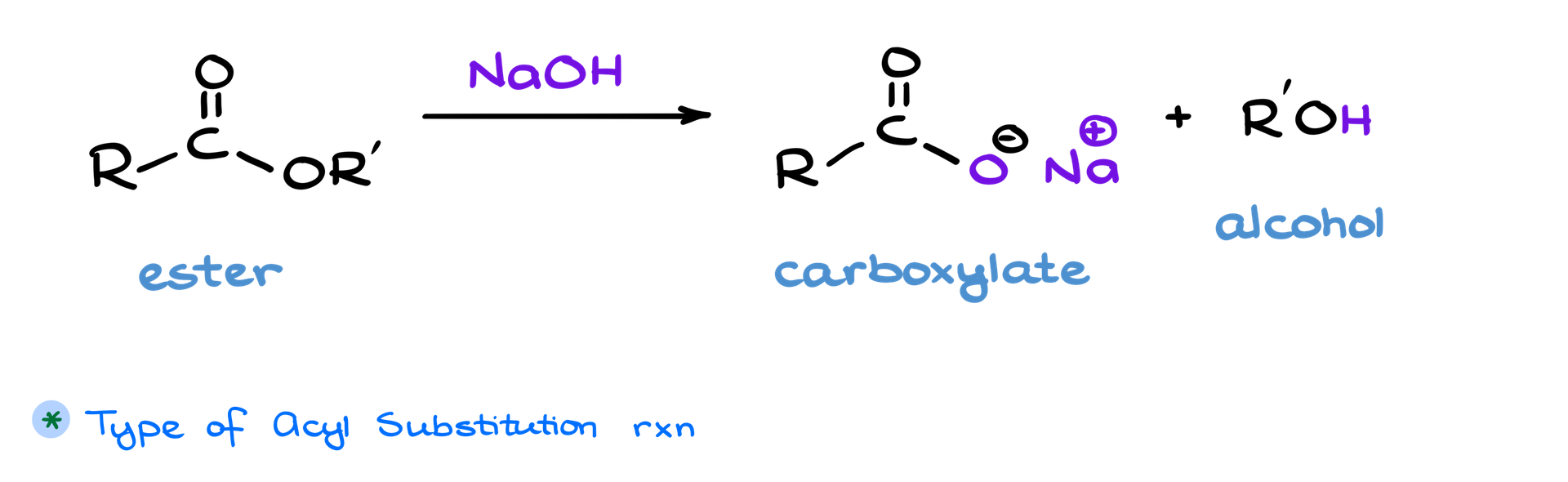
BTW, the name “saponification” comes from the Latin word sapo, meaning soap, because this reaction has historically been used to make soap. But there’s much more to saponification than its connection to soap—it’s a fundamental reaction in organic chemistry.
Saponification Mechanism
At its core, the reaction begins with the base, the hydroxide ion (OH⁻), attacking the carbonyl carbon of the ester. This attack forms what’s called a tetrahedral intermediate, a characteristic structure in reactions involving carboxylic acid derivatives. The carbonyl carbon, originally in a trigonal planar geometry, temporarily shifts to a tetrahedral geometry as the nucleophile adds to it.
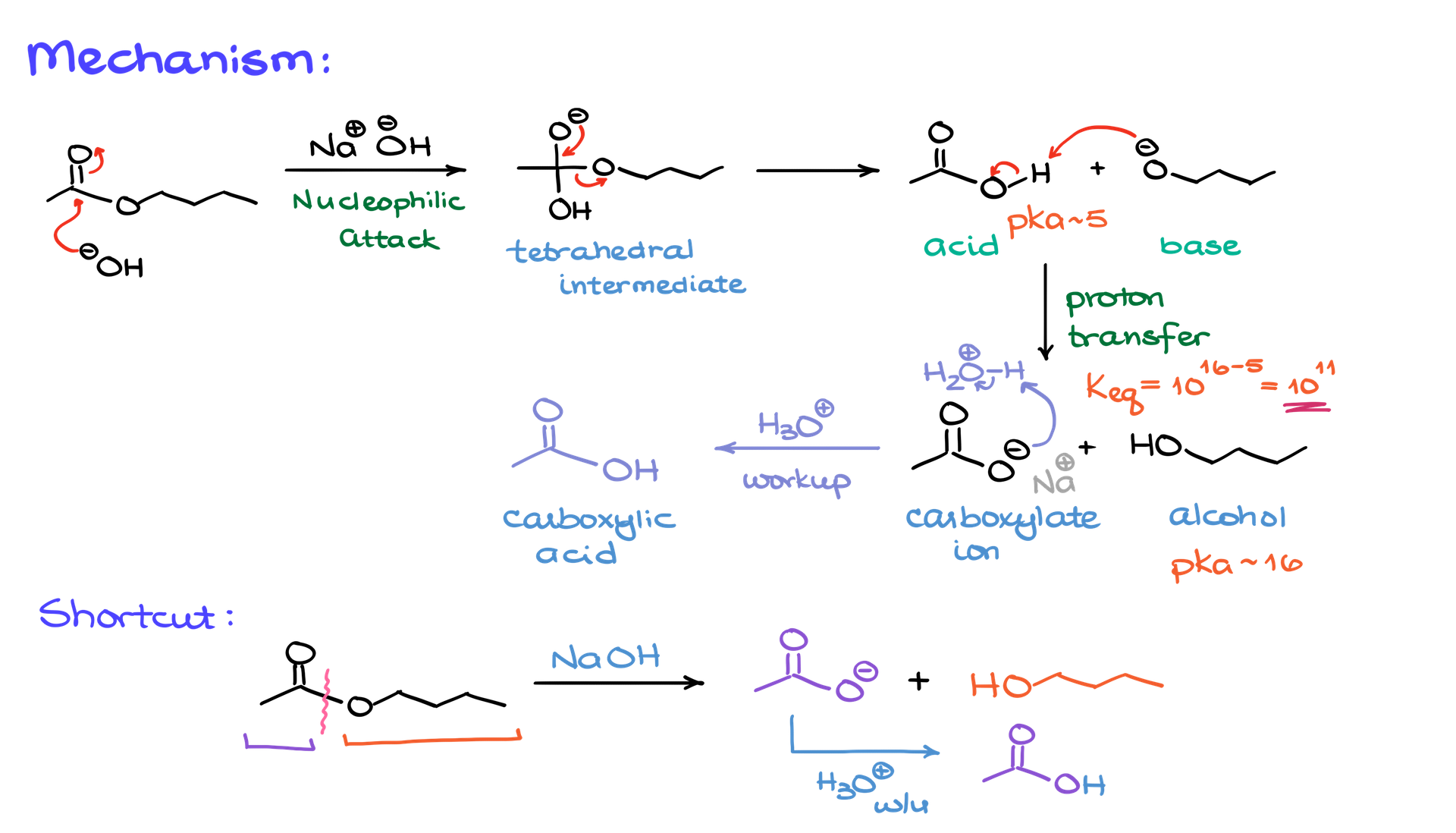
Next, the intermediate loses the leaving group, in this case, the alkoxide ion. This step produces two species: a carboxylic acid and an alkoxide ion. However, these two cannot coexist in the same solution because the alkoxide ion, being a strong base, immediately deprotonates the carboxylic acid. This creates a carboxylate ion and an alcohol. This proton transfer is not just a side reaction—it’s the driving force of saponification. The equilibrium for this step is heavily favored because the pKa of a carboxylic acid (about 5) is much lower than that of an alcohol (around 16). This vast difference in acidity pretty much means that the reaction progresses irreversibly.
While the carboxylate ion is a primary product, it’s often useful to convert it to the neutral carboxylic acid. This is done through an acidic workup, where an acid is added to protonate the carboxylate, giving the neutral acid as the final product. For example, if sodium hydroxide is used in the reaction, the carboxylate product will have sodium as the counterion. After the acidic workup, the final product will simply be the carboxylic acid.
Saponification Shortcut
The reaction mechanism may sound intricate, but there’s a simpler way to think about it. Imagine the ester bond as the connection between the carbonyl carbon and the oxygen of the alkyl group. In saponification, this bond breaks (see diagram above). The part of the molecule containing the carbonyl becomes the carboxylate, while the other part becomes the alcohol. For example, if butyl acetate undergoes saponification with sodium hydroxide, the products are sodium butyrate (carboxylate) and butanol (alcohol).
Examples of Saponification
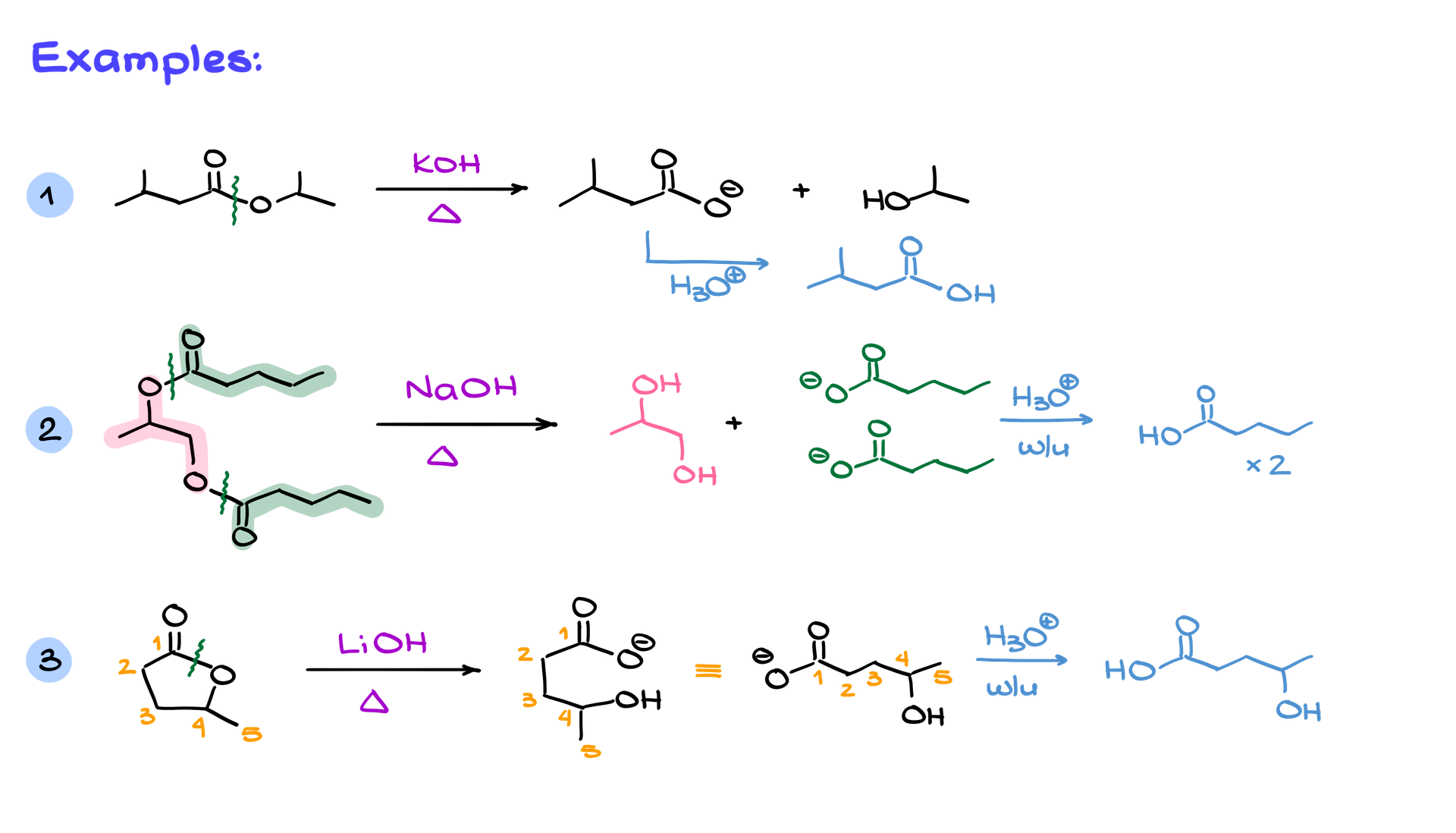
Saponification can be applied to more complex molecules as well. For instance, if a molecule has multiple ester groups, the reaction is not selective—each ester bond will hydrolyze, producing multiple carboxylates and alcohols. Even cyclic esters, known as lactones, undergo saponification. However, because the ester bond in a lactone is part of a ring, the reaction doesn’t create separate molecules. Instead, both the carboxylate and alcohol remain part of a single, linear product.
Though the term “saponification” might initially sound daunting, the reaction itself is straightforward. It’s a predictable and reliable way to transform esters into carboxylates and alcohols. With practice, understanding the steps of the mechanism becomes second nature. And since it’s a common reaction and is a part of many synthetic schemes, this one is definitely a must-know mechanism.
