Retro-Dieckmann Challenge Mechanism
In this tutorial, I have another awesome mechanism to share with you.
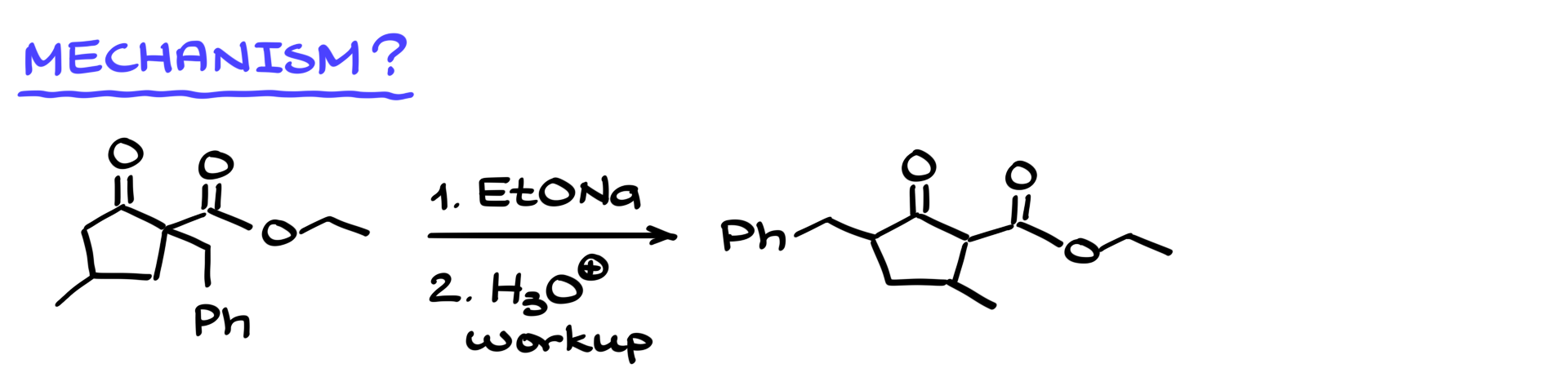
Looking at my starting material and final product, it’s clear that it seems like the benzyl group is shifting around, and the methyl group also appears to move. But of course, we know that simple rearrangements don’t allow alkyl groups to just hop around the molecule, unless it’s some sort of carbocation rearrangement. However, since we’re working under basic conditions, carbocations are unlikely to be involved here. What stands out, though, is the presence of two carbonyl groups in the starting material, with a quaternary position in between them. As we know from many reactions involving enols and enolates, having a quaternary position between two carbonyls can often trigger a retro-Dieckmann condensation or retro-Claisen condensation, depending on the nature of the starting material. Given that we’re working with a cyclic molecule, this is a retro-Dieckmann reaction.
So, let’s dive into it.
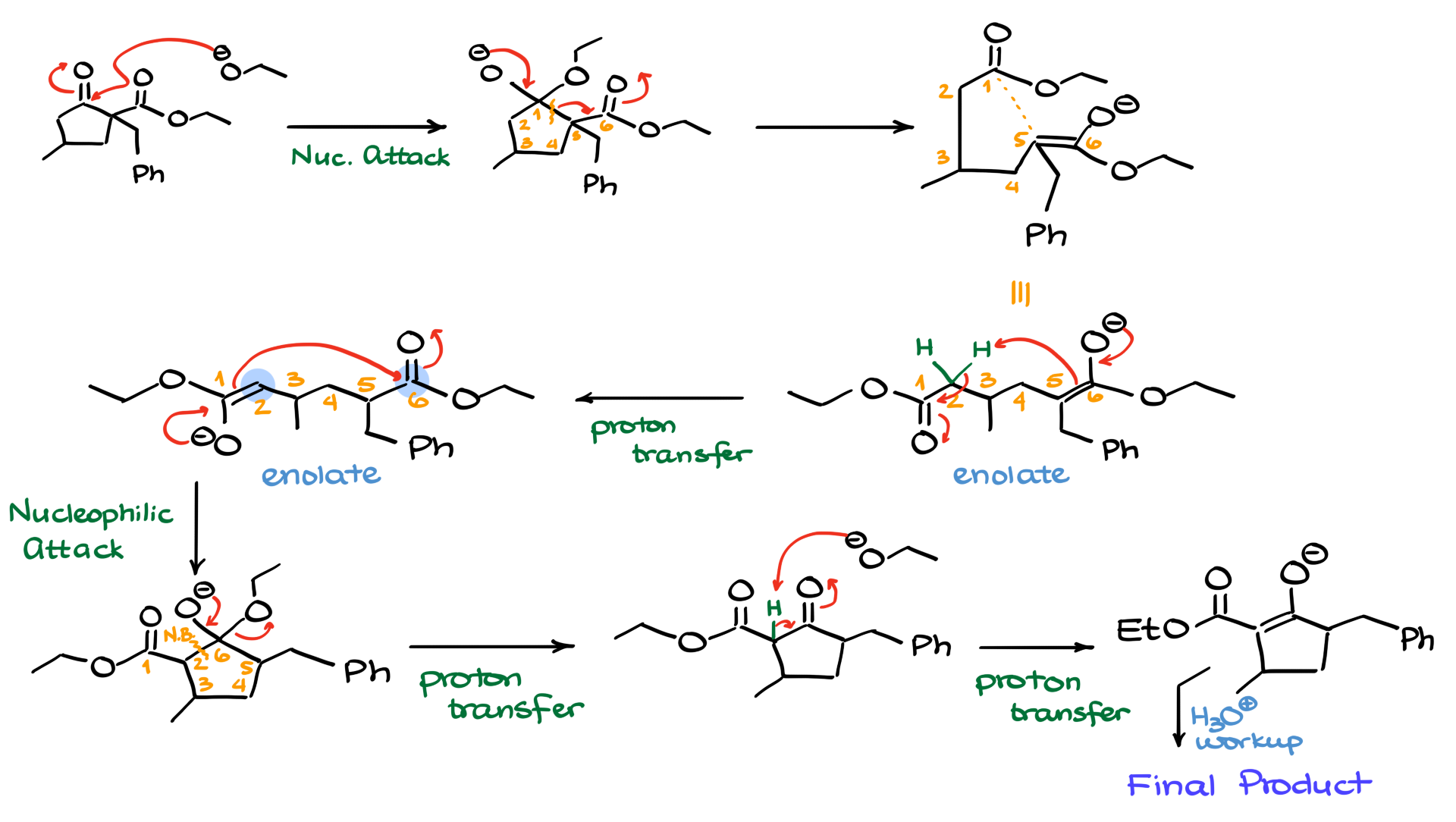
I’ll start by redrawing the starting material along with the ethoxide reagent we’re using. Between the two carbonyls, the ketone functional group is going to be more electrophilic. That means our first step is a nucleophilic attack on the ketone, giving us the intermediate you see here. Next, the oxygen pushes electrons down, breaking the bond between the carbons—let’s call them carbon number 1 and carbon number 5, with carbon number 6 also playing a role here. As the molecule opens up, we get this intermediate, which, yes, I’ve drawn with some pretty atrocious bond angles, but that’s just to emphasize that the bond that used to exist between these atoms is now gone. The atoms 1 through 6 are still in place, as indicated.
If we redraw the molecule with more reasonable bond angles, we get the open-chain version, and numbering the atoms makes it so much easier to keep track of where everything is, so we don’t lose any atoms or misplace any groups. For example, carbon number 3 still holds the methyl group, and the benzyl group sits on carbon number 5 in this open-chain structure. The intermediate we have here is, of course, an enolate, but simply closing it back up in a Dieckmann condensation would just lead us back to the starting material, and we know we’re heading in a different direction. The key is recognizing that there’s another alpha position at carbon number 2, which can act as a nucleophile.
This re-enolization step, shown here as an intramolecular process, could spark a debate in the comments below about whether it’s truly intramolecular or if we’d need a “chaperone” for the proton transfer. But for the sake of simplicity, I’ll show it like this. Regardless of how you prefer to illustrate that proton transfer, the next intermediate we get is an enolate where the left side of the molecule is now nucleophilic, ready to react with the electrophilic right side. Showing the curved arrows, we see electrons flowing from the nucleophile to the carbonyl, pushing electrons onto the oxygen, forming a new carbon-carbon bond between carbon number 2 and carbon number 6.
Let’s clear some space for the next steps. With those electrons flowing as shown, the bond between carbons 2 and 6 forms, giving us a five-membered ring as the intermediate. That new bond is squeezed right in here between carbons 2 and 6. Next comes the dissociation of the leaving group from our tetrahedral intermediate, with oxygen kicking out the ethoxy group, leaving us with a dicarbonyl and the expelled ethoxide. At this stage, we immediately deprotonate the position between the carbonyls, which drives the reaction forward and gives us the enolate intermediate. Finally, to wrap things up, we neutralize the molecule with an acidic workup, protonating the enolate to form our final product, which looks just like the intermediate we had in the middle of the mechanism. I just ran out of screen space to show the final product, but you know exactly what it is.
So, what did you think about this mechanism? Did you manage to figure it out, or did it leave you completely lost? Let me know in the comments below!
