Retro-Aldol Challenge Mechanism
I have a really fun question here for you.
So, we are starting with this four-membered ring containing an OH group and a ketone, so it kind of looks like an aldol. And we are making, through some sort of magic, this six-membered ring with a double bond and a ketone, which would be an alpha-beta unsaturated compound. The question is: can we figure out a reasonable curved arrow mechanism for this?
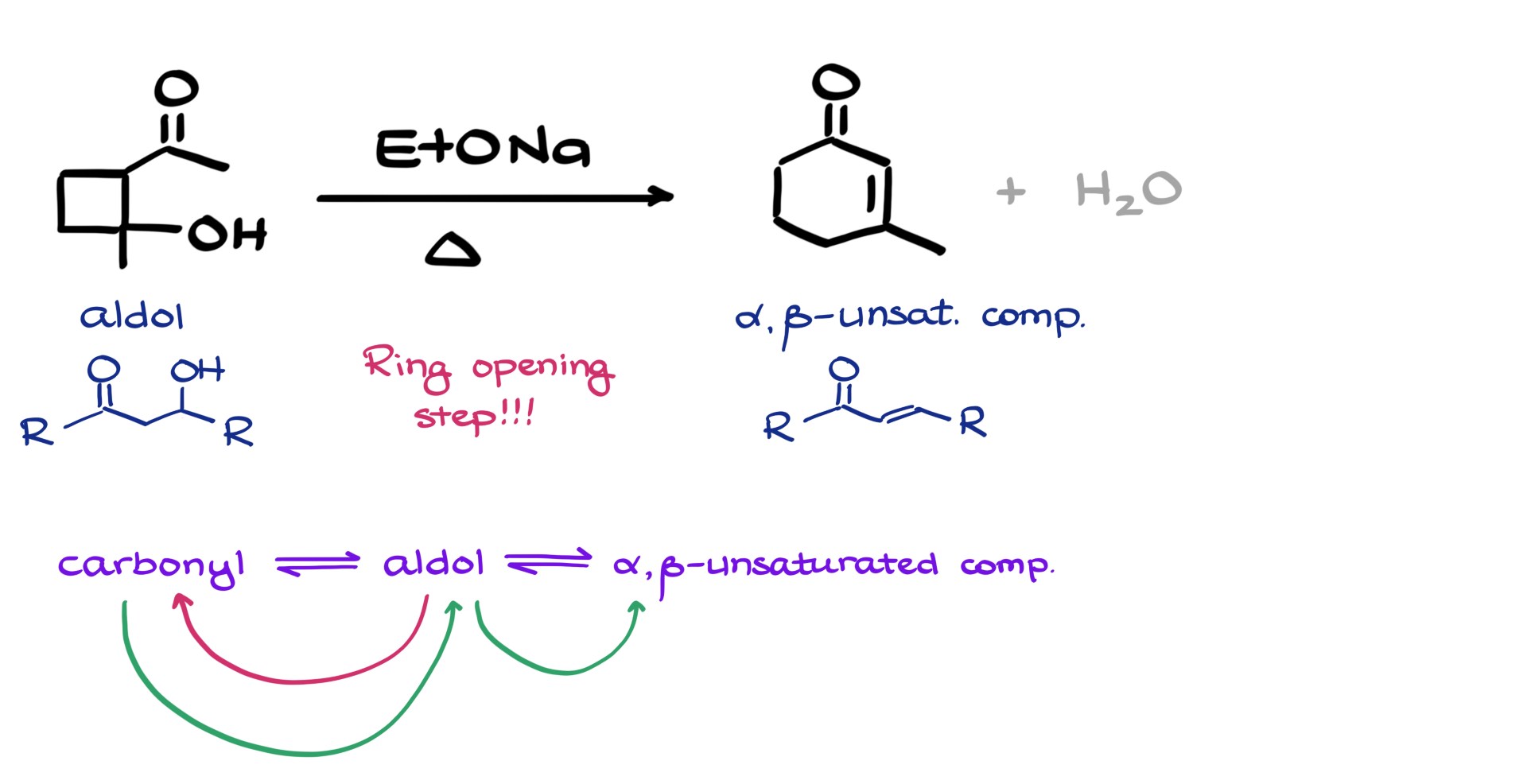
When it comes to this question, the very first thing that jumped out at me right away is that I have the aldol starting material, although in this case, we have a cyclic aldol, so we would have a cycle sitting something like that. I also have an alpha-beta unsaturated compound here, which also looks like a cycle. Another thing to remember is that both of these structures are part of the aldol condensation mechanism. However, in this case, I cannot directly go from my aldol to my alpha-beta unsaturated compound because structurally, we start with a four-membered ring and finish with a six-membered ring, which means we are looking at a ring-opening step somewhere in our mechanism that we will then close back up again to make our final product.
So, the idea for my mechanism here would be to start with the aldol and form an open-chain carbonyl, and then from that carbonyl, form a different aldol from which I will make my final alpha-beta unsaturated compound. Since every step in the aldol condensation reaction is in equilibrium, I can just use the template for the aldol condensation and go in whichever direction I need to in order to get to, well, where I need to go. Now that we have the general idea for our mechanism and how we need to proceed, let’s give it a try.
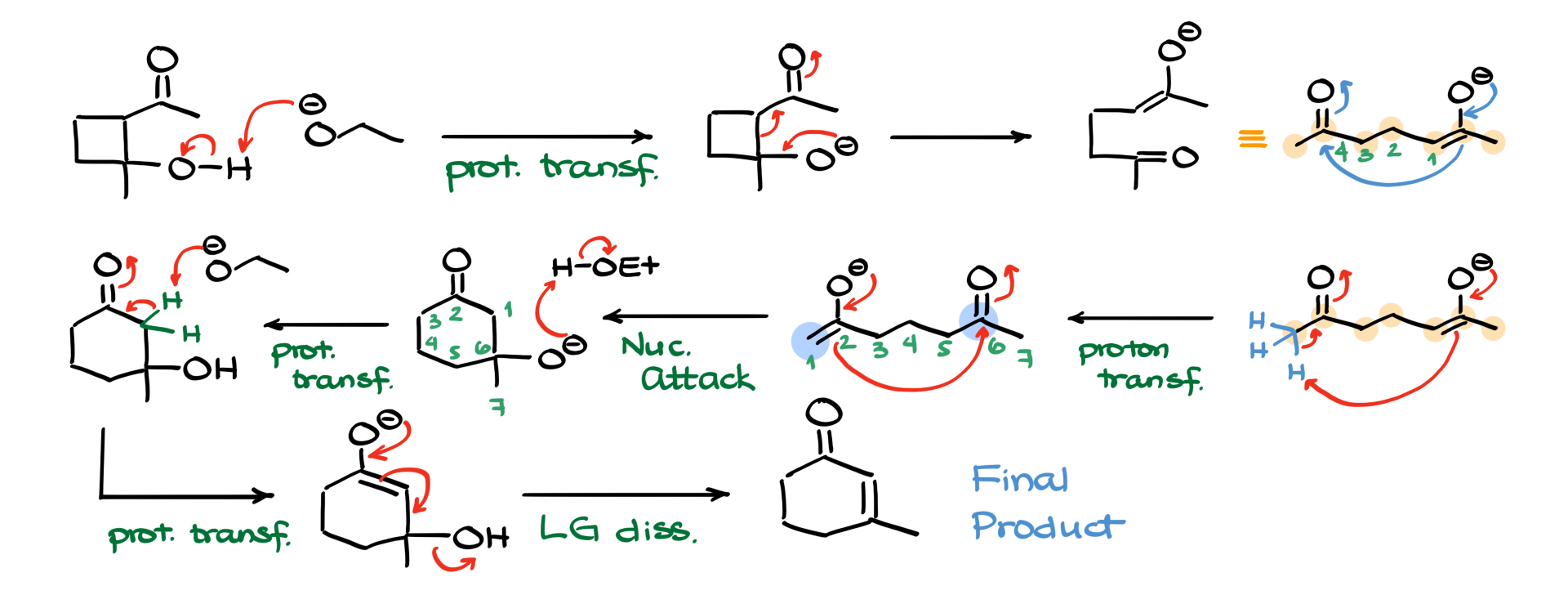
I’m going to start by drawing my starting material and the ethoxide base. The very first step I’m going to do here is a proton transfer, deprotonating my molecule at the OH group and forming the corresponding negatively charged intermediate. Now, if we were doing the aldol condensation reaction, the preceding step to this one would have been the formation of this new carbon-carbon bond, which means that now I need to go back and break this bond. To do that, I’m going to have my electrons from the oxygen go down, breaking that bond and essentially making an enolate species that looks like this. I am purposefully drawing this molecule like this, with horrible bond angles, so it is easier to see what we have changed here.
If I were to redraw this molecule in a slightly better linear fashion, I would end up with a seven-carbon chain looking like this. Counting the carbons in this chain, I see that I have one, two, three, four, five, six, seven carbons, which means that in order to re-close this ring into a six-membered ring, I can no longer do this because that would bring me back to my four-membered ring. So, to close my six-membered ring, I have to re-analyze a different position in my molecule. I will redraw my molecule and, instead of doing the nucleophilic attack, I’m going to do the proton transfer that essentially recreates the enolate on the other side of my molecule, looking like this.
Now, if I were to do my nucleophilic attack on the carbonyl like so, I would end up making a new carbon-carbon bond between this carbon of my enolate and the carbon of my carbonyl, forming a six-membered ring over here, looking like this. If I wanted to renumber my atoms, I would have my carbons one, two, three, four, five, and six like that. And, I guess for completeness’ sake, we can number our carbon seven and indicate it over here as well.
The next step in our mechanism, since we are in basic media, is to protonate our negatively charged species with our solvent, which is ethanol. So, oxygen is going to grab this proton from ethanol, making another aldol intermediate and, of course, the ethoxide that we have just generated. From this point, we are going to re-analyze our molecule by pulling one of these protons off from the alpha position, making the corresponding enolate. The only thing left for us is to kick our leaving group out, giving us our final product. Easy peasy.
Reactions like this are a common guest on final exams, so make sure you practice your mechanisms to the point where you can write them in both directions. By the time the exam comes, you’ll be all ready, and nothing can scare you.
