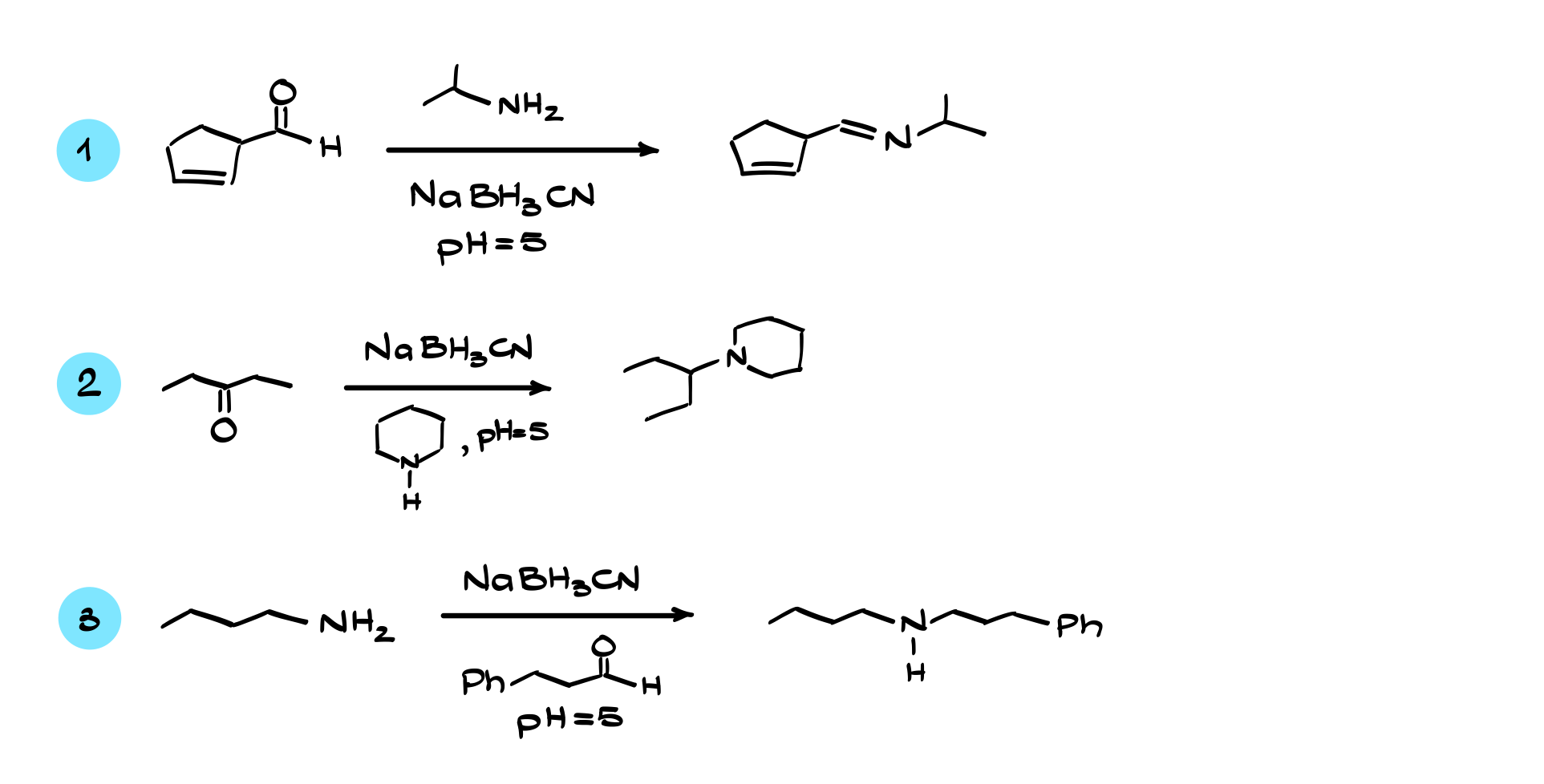
Reductive Amination
In this tutorial, I want to talk about reductive amination, one of the most widely used and flexible methods of amine synthesis.

General Scheme of Reductive Amination
The general idea of reductive amination is fairly straightforward and simple.
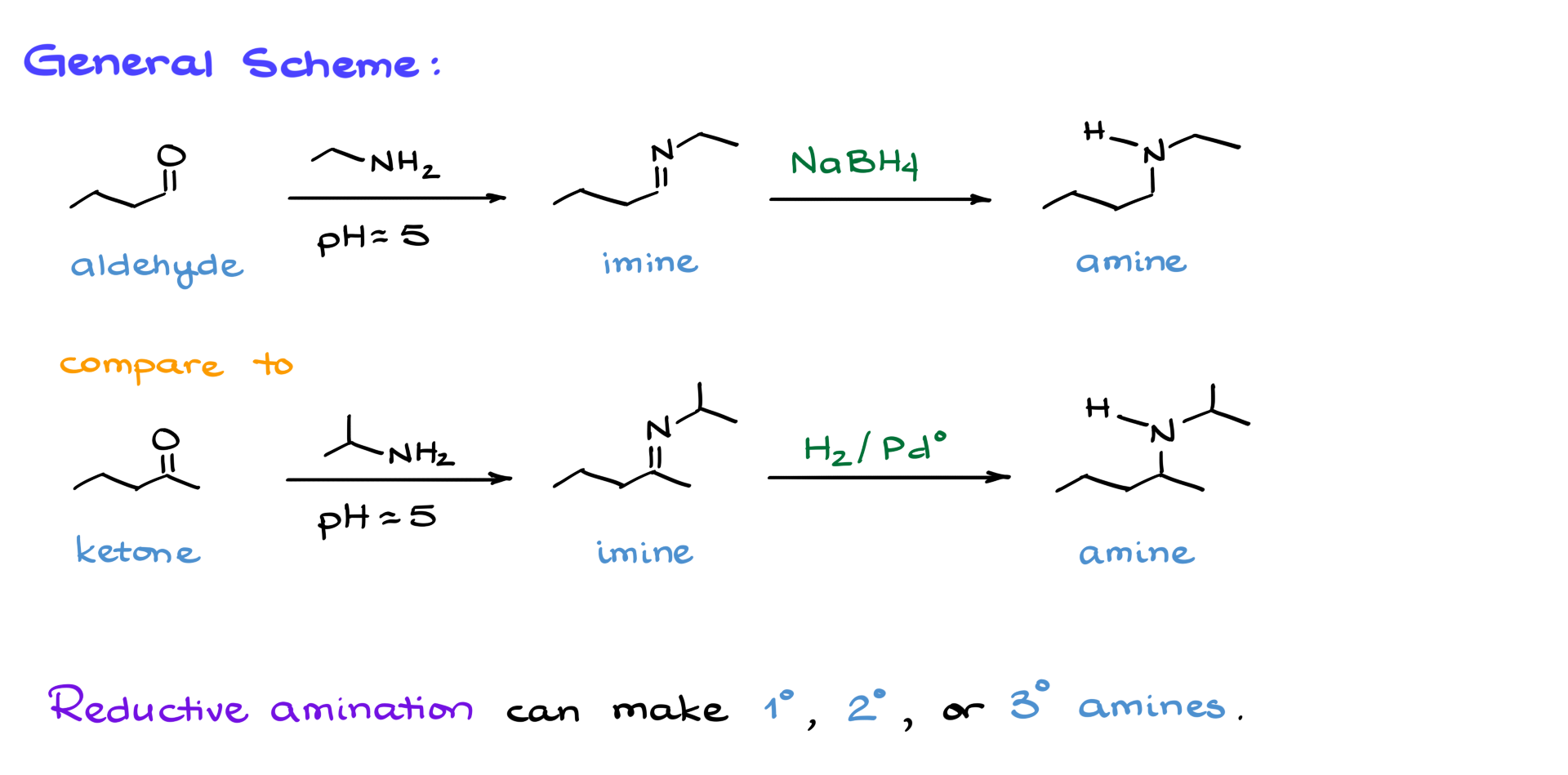
We start with a carbonyl compound, either an aldehyde or a ketone, and react it with an amine—typically a primary or a secondary amine. This reaction is usually performed under slightly acidic conditions, requiring just a small amount of acid for catalysis. That’s why I’m showing a pH of 5 here, indicating that we are barely acidic. At this stage, we obtain the corresponding amine intermediate. However, that is not our final product. Once we have this intermediate, we proceed by reducing the carbon-nitrogen double bond to obtain the corresponding amine.
The best part about reductive amination is its flexibility—it can produce primary, secondary, or even tertiary amines depending on the nature of the starting materials. This makes it an extremely versatile method capable of yielding almost any amine imaginable. So, let’s take a closer look at the mechanism of this reaction.
Reductive Amination Mechanism
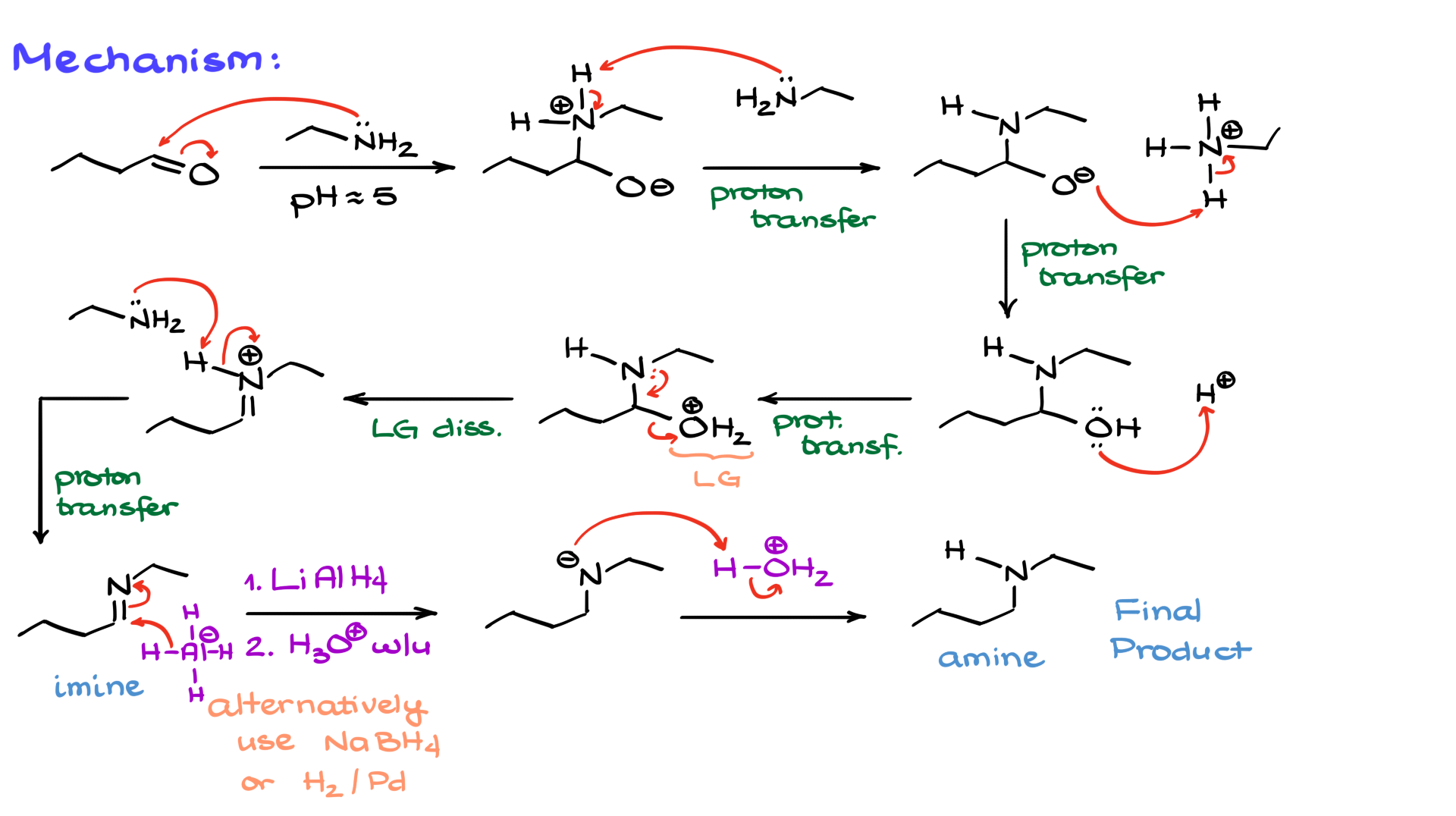
The first part of the mechanism involves imine formation. To illustrate this, I will use butanal (an aldehyde) and ethylamine as my starting materials. Step one in imine formation is the nucleophilic attack of the nitrogen onto the carbonyl, forming an intermediate. Sometimes, you might see the carbonyl being protonated first, creating a positively charged intermediate where the oxygen has already been protonated, forming an OH group instead of O-. I go into more detail about that in my tutorial on imine formation and hydrolysis, so check that out if you want a deeper explanation.
Next, I introduce a second equivalent of amine, which serves to deprotonate the intermediate at the nitrogen. At the same time, I protonate the oxygen, yielding the hemiaminal intermediate. And as I mentioned earlier, the order of these proton transfers doesn’t really matter—if you see your instructor or textbook presenting them in a slightly different order, that’s perfectly fine.
In the next step, we need to convert the OH group into a good leaving group, which is where our trace amount of acid comes into play. The acid protonates the OH, turning it into a better leaving group. The nitrogen then facilitates the departure of this leaving group, forming a protonated iminium ion. The final step in this phase is deprotonation of the iminium ion. Here, we use our second equivalent of amine to remove the proton, forming the imine intermediate. This marks only the halfway point in our reaction, as we now move on to the second step: the reduction of the imine.
When it comes to reduction, we have multiple options. For this mechanism, I will use lithium aluminum hydride (LAH) as my reducing agent, but we could also use sodium borohydride or even catalytic hydrogenation with palladium or platinum. Reduction with complex hydrides typically works best, but in some cases, hydrogenation might be necessary, especially if there are functional groups that are sensitive to complex hydrides. The choice of reducing agent depends on the specific functional groups in your molecule.
To show this part of the mechanism, I depict the aluminum hydride attacking the carbon of the C=N double bond, effectively adding a hydride (a hydrogen with two electrons) to the carbon. This results in a negatively charged intermediate, which we then protonate in an acidic workup to form our final, neutral amine product.
Single-Pot Reductive Amination Procedure
Now, while this two-step process—first forming the imine and then reducing it—is not particularly complicated, we can make it even more efficient. Instead of running two separate steps, we can use a one-pot or single-pot procedure, where both imine formation and reduction happen in the same reaction vessel.
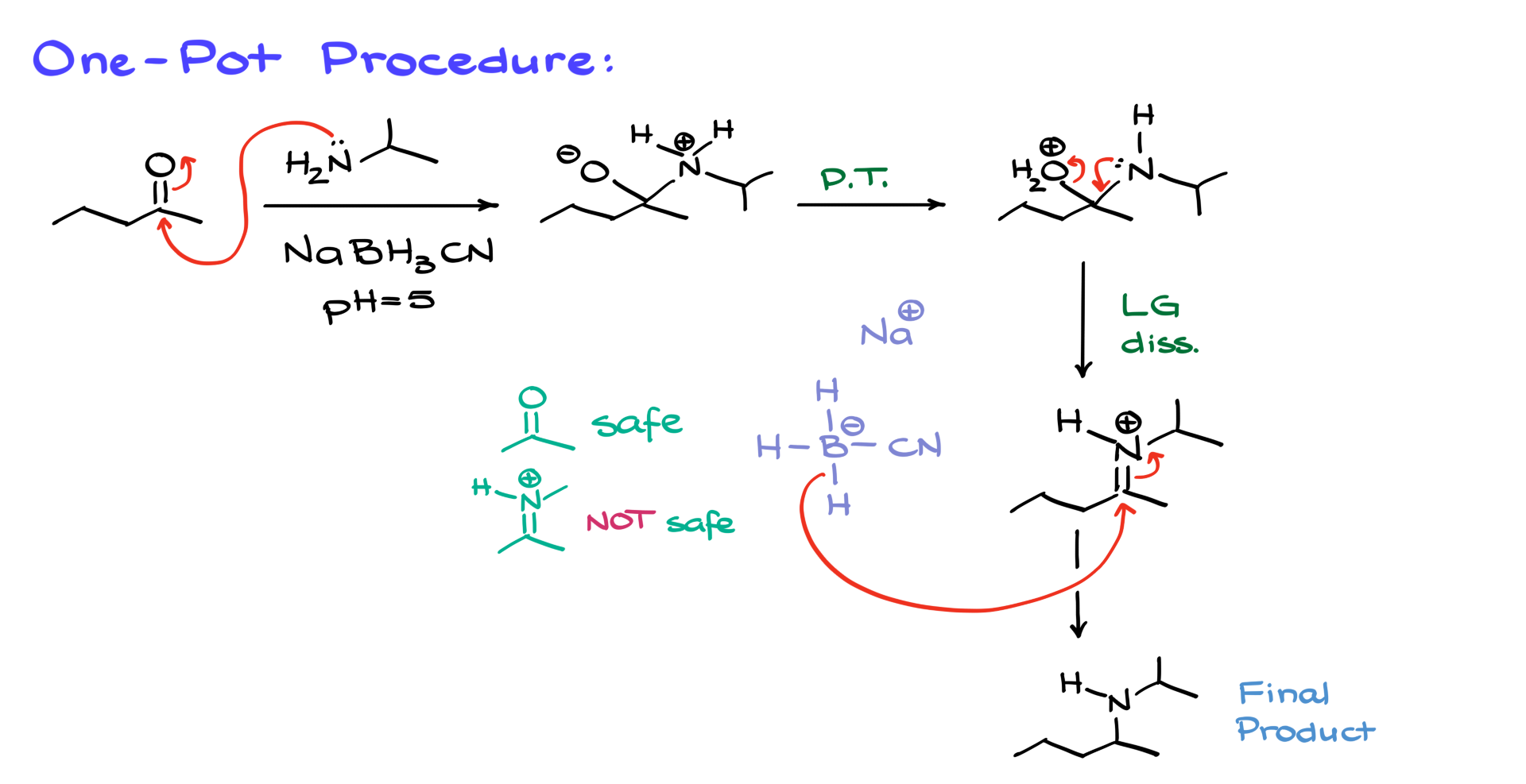
For a one-pot procedure, we slightly modify our conditions. We still start with a ketone or aldehyde and an amine, but our reducing agent is specifically sodium cyanoborohydride (NaBH3CN). The cyano group on this reagent plays a crucial role. Regular sodium borohydride would reduce the ketone immediately, preventing imine formation. However, sodium cyanoborohydride is less reactive and does not reduce ketones efficiently, giving the amine enough time to react with the carbonyl first.
The reaction mechanism begins as before, with nucleophilic addition of the nitrogen to the carbonyl, forming a charged intermediate. After a series of proton transfers, we reach the point where the leaving group departs, generating the iminium ion. Here’s where the fun part comes in: sodium cyanoborohydride is selective. While it does not react quickly with carbonyls, it readily reduces iminium ions because the positive charge on nitrogen makes them more electrophilic than carbonyls. As soon as the iminium ion forms, sodium cyanoborohydride immediately reduces it, yielding the final amine product in a single step.
On paper, drawing one extra step in your synthesis might not seem like a big deal, but in the lab, every extra step can mean hours—or even a whole day—of additional work. So, saving time with a one-pot procedure is incredibly useful. I don’t know about you, but I’d much rather finish early and enjoy a cup of tea with a good book than spend unnecessary hours in the lab. Because of this, the one-pot reductive amination method with sodium cyanoborohydride is the most common approach you’ll see in your coursework. While there are other reagents that can accomplish the same transformation, sodium cyanoborohydride is by far the most widely used, so for now, we’ll focus on that.
Reductive Amination Example 1
Now that we understand the mechanism and the procedure, let’s go through some examples.
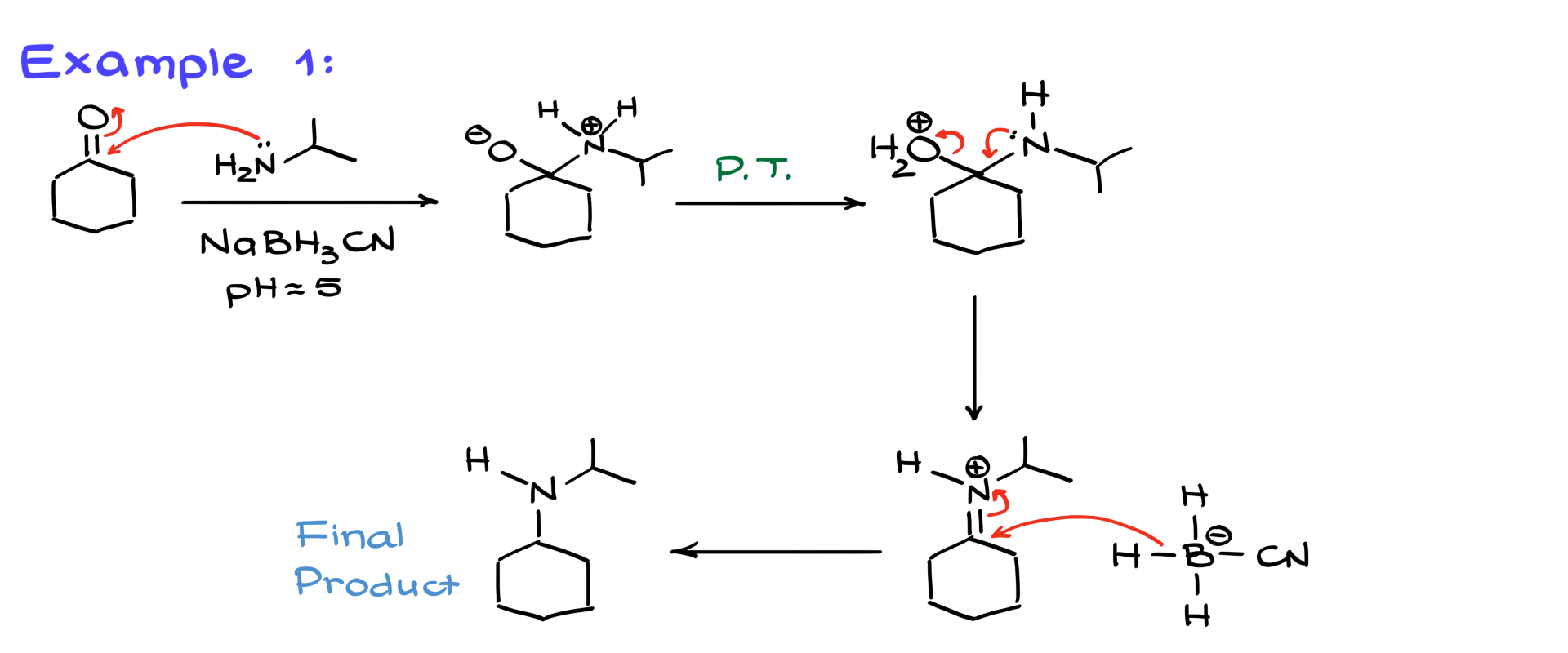
First, we have a reaction between cyclohexanone (a cyclic ketone) and isopropylamine in the presence of sodium cyanoborohydride. Since we are using NaBH3CN, we know this is a one-pot procedure. Mechanistically, the first step is nucleophilic attack on the carbonyl, forming the charged intermediate. Then, after proton transfers, the leaving group is expelled, generating the iminium intermediate. At this point, sodium cyanoborohydride reduces the iminium ion, yielding our final amine product. Simple, right?
Reductive Amination Example 2
Let’s look at another example.
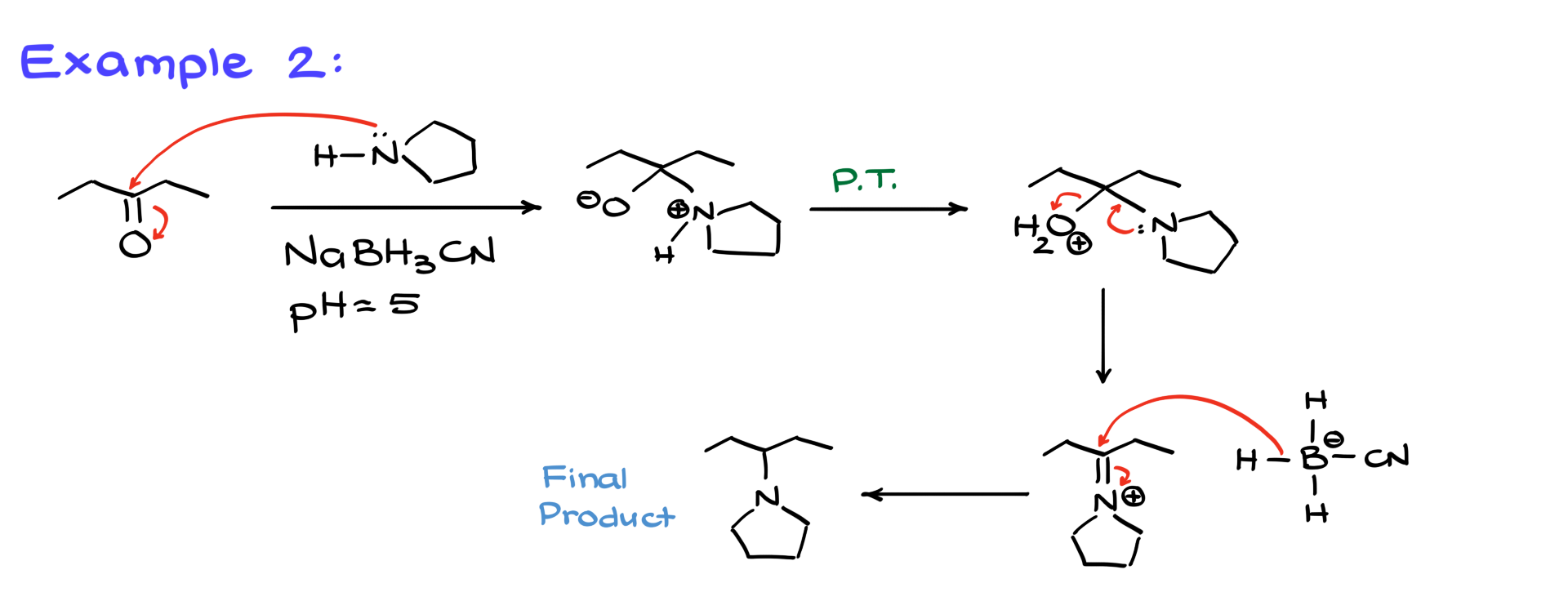
Here, we have a ketone reacting with a cyclic amine, again in the presence of sodium cyanoborohydride. The mechanism remains the same. The nitrogen attacks the carbonyl, forming an intermediate, followed by proton transfers and the departure of the leaving group, which creates the iminium ion. Since we’re not trying to make a stable imine, the cyanoborohydride immediately reduces it, producing our final product—a tertiary amine in this case.
Reductive Amination Example 3
Now, here’s a more interesting example.
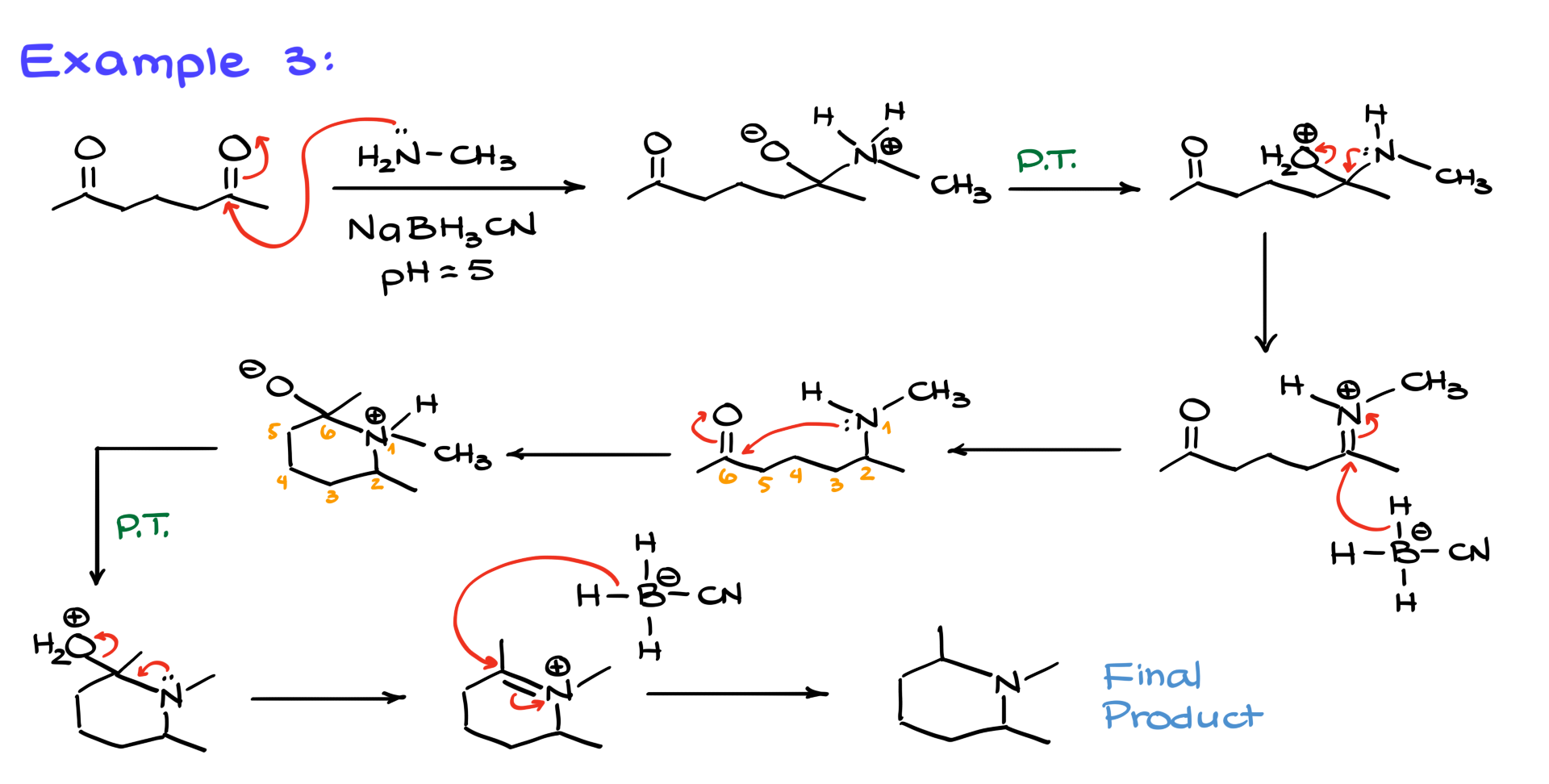
This time, we have a molecule with two carbonyl groups. As before, the reaction starts with nucleophilic attack by the amine, forming a charged intermediate. Proton transfers follow, leading to the departure of the leaving group and the formation of an iminium ion, which is then reduced. So far, so good, right?
But here’s the twist: once the imine is formed, we still have a carbonyl present in the molecule. Since the imine nitrogen and the carbonyl are part of the same molecule, they are perfectly positioned to react with each other, forming a six-membered ring. This intramolecular reaction happens spontaneously because nitrogen is a great nucleophile, and carbonyls are excellent electrophiles.
After the ring forms, another round of proton transfers leads to the formation of a cyclic iminium ion, which is then reduced by sodium cyanoborohydride, giving us a cyclic amine as the final product. So, if you like organic structures, just put a ring on it!
Practice Questions
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!