Reduction of Carboxylic Acids and Derivatives with Complex Hydrides
In this tutorial, I want to talk about the reduction of carboxylic acids and carboxylic acid derivatives using complex hydrides like lithium aluminum hydride.

First, let’s look at the general scheme for this reaction. Here, I have the generic formula for a carboxylic acid derivative where X represents the leaving group. If the leaving group is chlorine, we have an acid chloride; if it’s an alkoxide, we have an ester, and we can also consider the carboxylic acid itself.
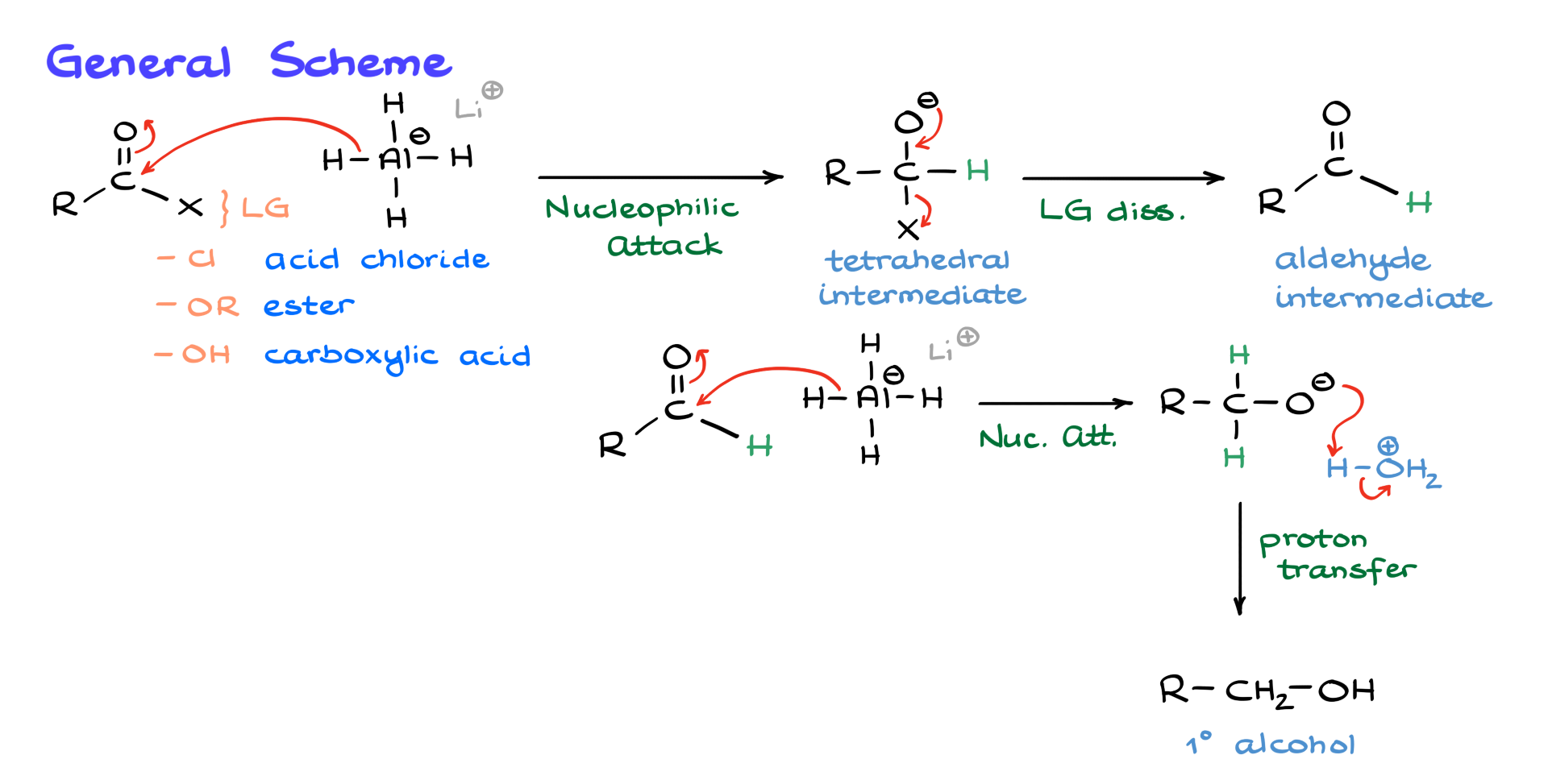
The general mechanism works as follows: lithium aluminum hydride, our reducing agent, serves as a source of H⁻, which will attack the carbonyl, pushing the electrons onto the oxygen atom. As a result of this nucleophilic attack, we obtain the corresponding tetrahedral intermediate. This intermediate will immediately lose the leaving group as the electrons push back onto the carbonyl carbon, kicking X out and giving us the aldehyde intermediate. Since we are still in the presence of excess lithium aluminum hydride, the aldehyde undergoes a second nucleophilic attack by another equivalent of hydride.
Before we continue, I want to make a quick disclaimer. For simplicity, I will keep rewriting lithium aluminum hydride in a straightforward form. In reality, the system contains a variety of reducing agents involving aluminum hydrides, but we don’t need to dive into those details. The important thing to remember is that these reactions always use an excess of lithium aluminum hydride, meaning we have as much of the reagent as needed throughout the reaction.
Returning to the nucleophilic attack, the result is an alkoxide intermediate, and since we cannot go any further with reduction, we proceed with an acidic workup. Adding water and acid allows for proton transfer, giving us the final product, which in this case is a primary alcohol.
Now that we’ve covered the general mechanism, let’s apply it to some specific examples.
Reduction of Acid Chlorides
For our first example, let’s look at the reduction of an acid chloride.
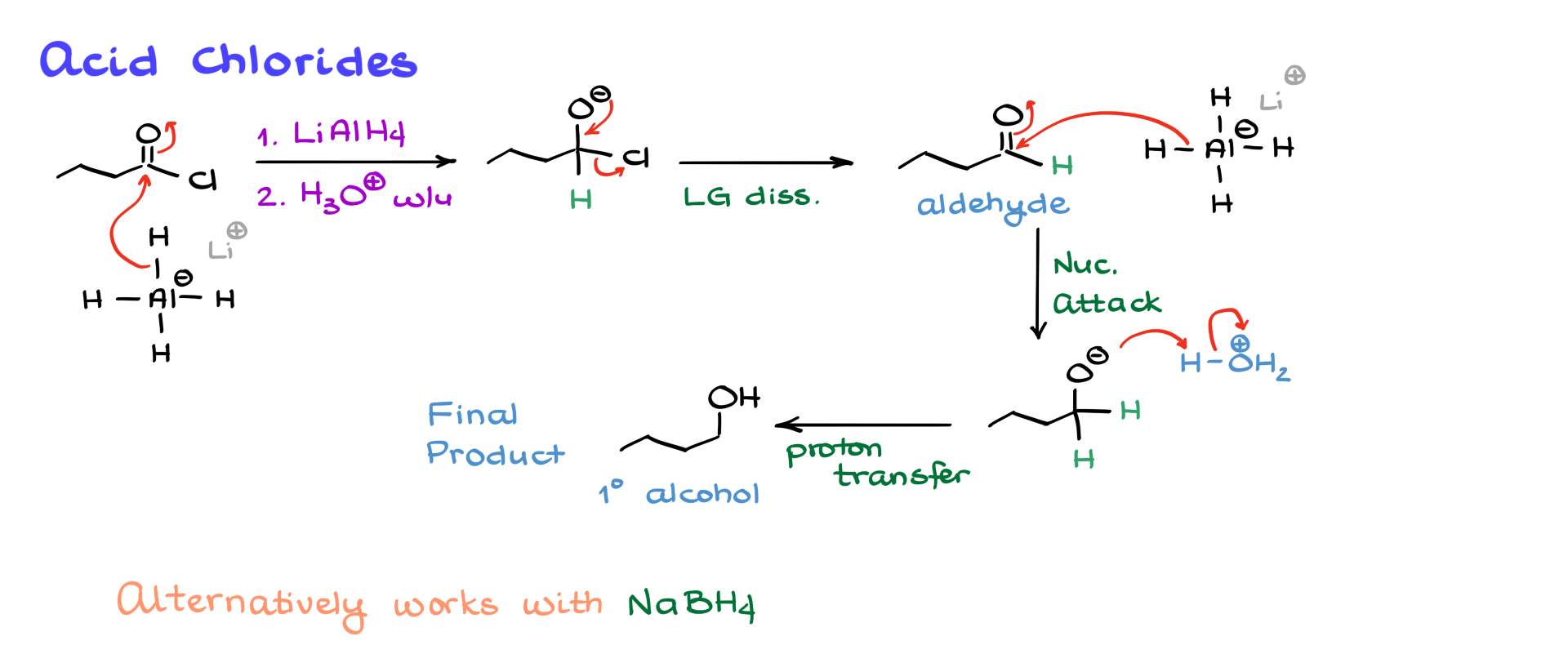
The first step involves lithium aluminum hydride performing a nucleophilic attack on the carbonyl, forming a tetrahedral intermediate. This intermediate then expels the chloride leaving group, yielding an aldehyde. Since we are using excess lithium aluminum hydride, the aldehyde undergoes a second nucleophilic attack, producing an alkoxide intermediate. The final step is an acidic workup, protonating the alkoxide and yielding a primary alcohol.
Acid chlorides are extremely electrophilic, which means that even a mild nucleophile like sodium borohydride can reduce them. While sodium borohydride does not work for most carboxylic acid derivatives, it is sufficient for reducing acid chlorides to alcohols.
Reduction of Esters
Now, let’s move on to esters.
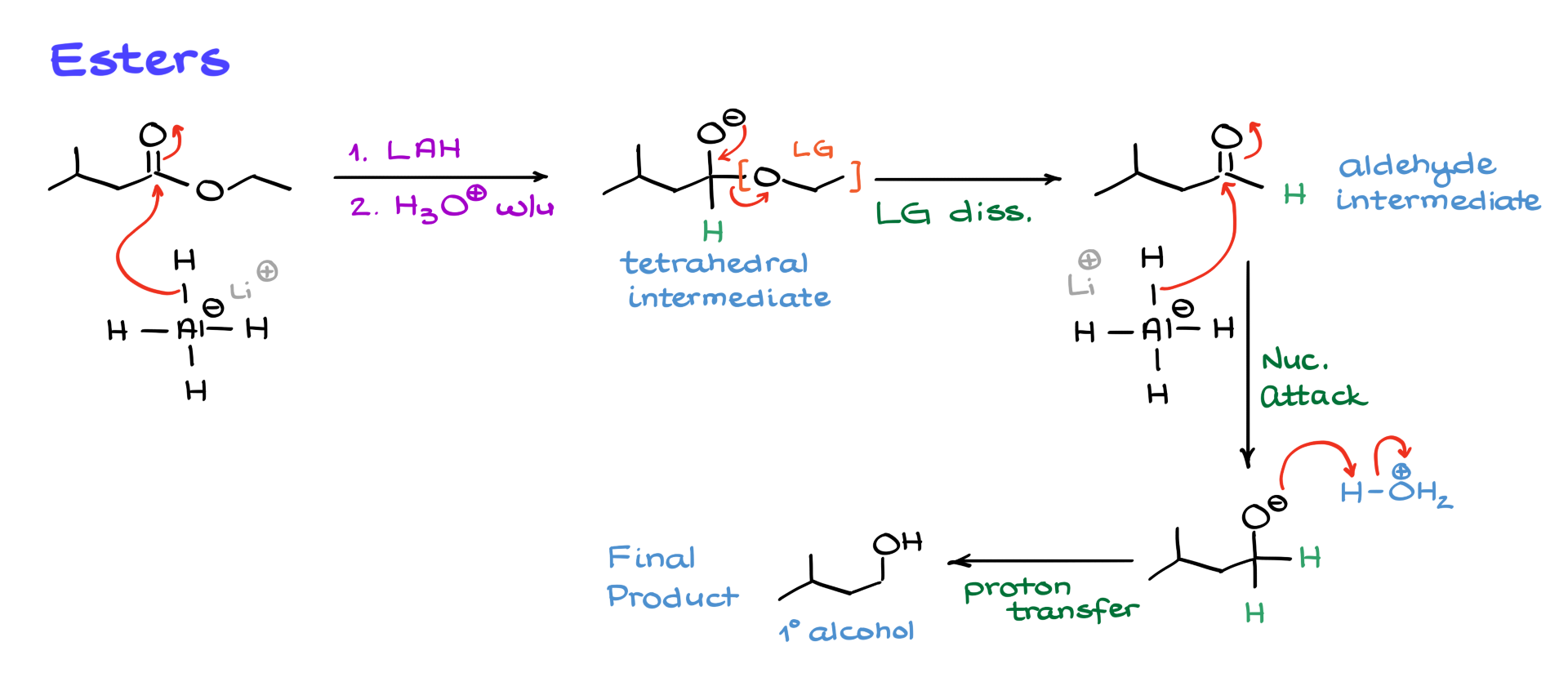
As before, lithium aluminum hydride attacks the carbonyl, forming a tetrahedral intermediate. The leaving group, which is the alkoxide portion of the ester, is expelled, yielding an aldehyde. The aldehyde then undergoes a second reduction step, forming an alkoxide, which after acidic workup, gives a primary alcohol.
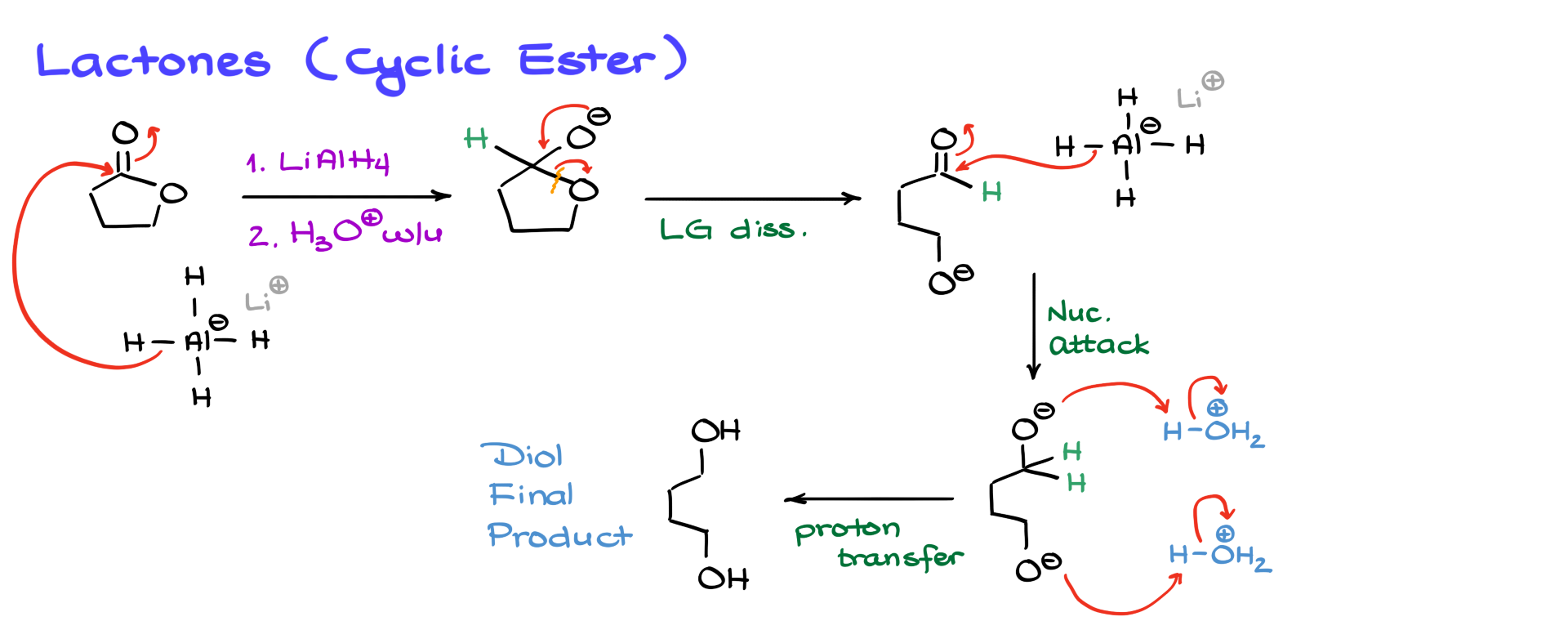
However, things become a bit more interesting when dealing with cyclic esters, or lactones. In this case, the same mechanism applies, but since the leaving group is part of the molecule itself, breaking that bond results in the opening of the ring. The aldehyde formed then undergoes a second reduction, leading to a diol as the final product. When working with lactones, remember that instead of completely breaking the molecule apart, you are opening the ring, and both ends will contain hydroxyl (-OH) groups.
Reduction of Carboxylic Acids
Next, let’s discuss the reduction of carboxylic acids.
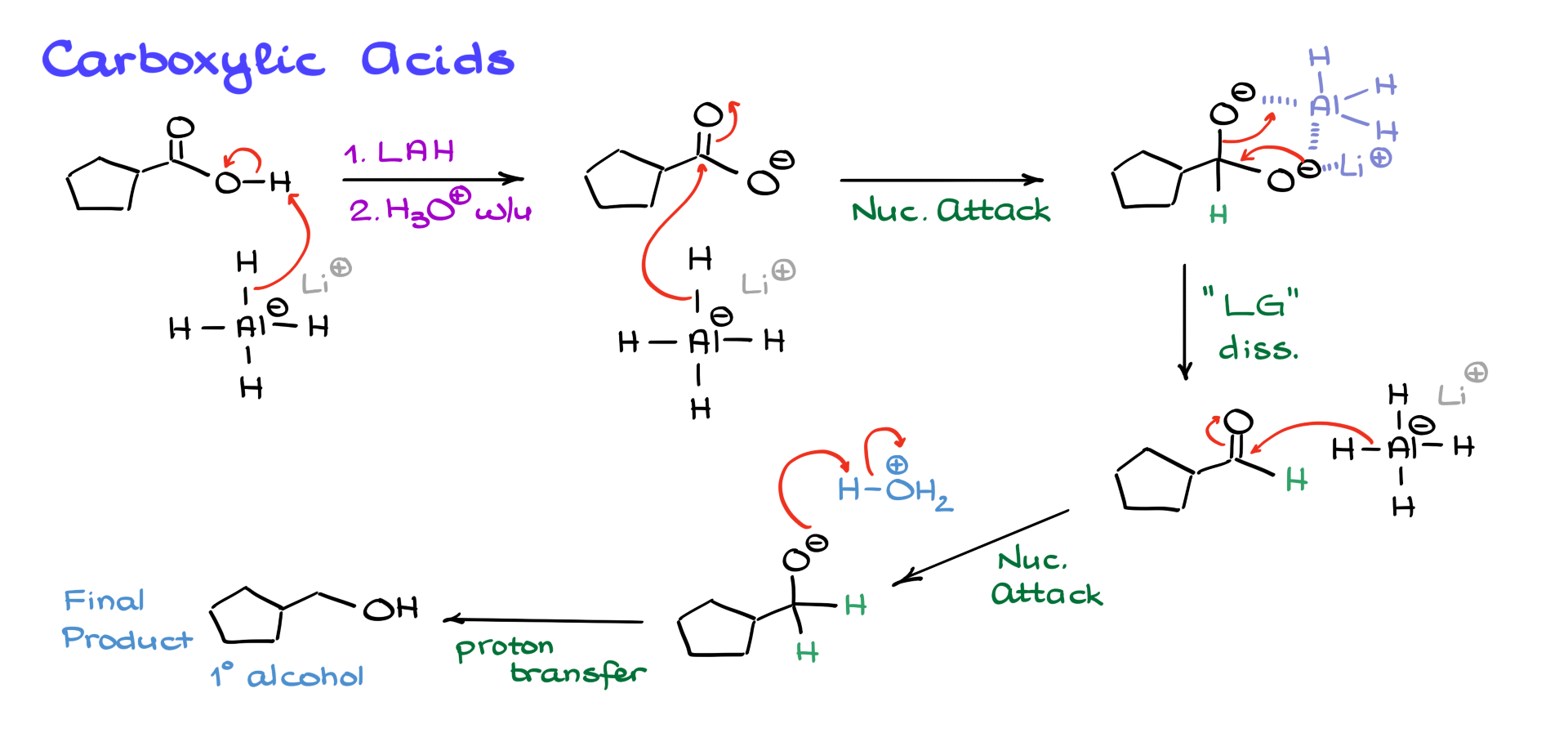
When lithium aluminum hydride is introduced to a carboxylic acid, the first reaction that occurs is a proton transfer. Lithium aluminum hydride is a strong base, so it readily deprotonates the carboxylic acid, forming a negatively charged intermediate. This reaction is highly exothermic and potentially dangerous, requiring careful handling.
Despite the negative charge, lithium aluminum hydride is powerful enough to attack the carbonyl, forming a doubly negatively charged intermediate. Research shows that aluminum and lithium coordinate with the oxygens, forming a complex structure, but for simplicity, we can imagine one of the oxygens acting as a leaving group. This results in the formation of an aldehyde, which undergoes further reduction to yield a primary alcohol after acidic workup.
Reduction of Amides
Things change when we introduce nitrogen-containing functional groups, such as amides.
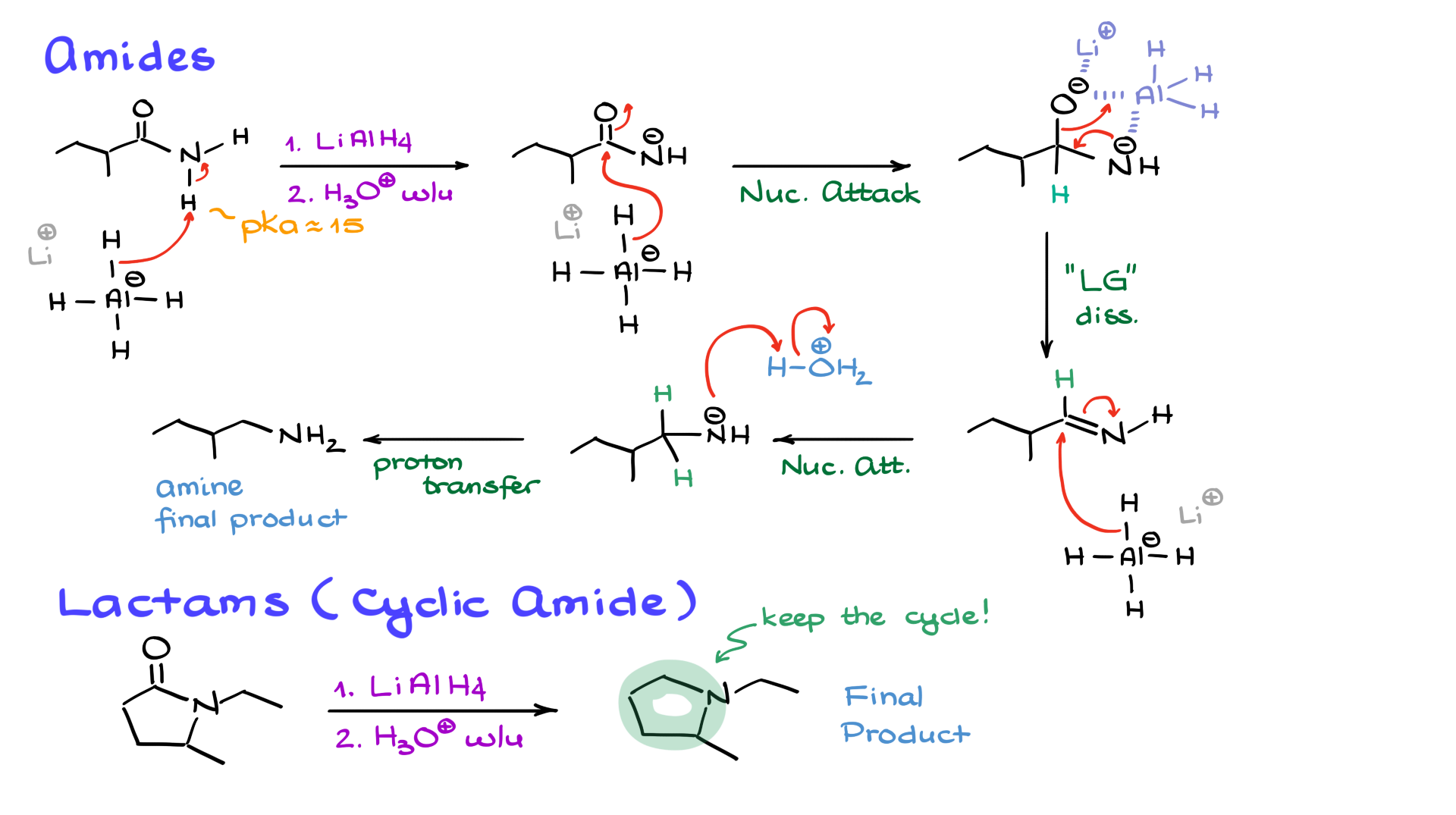
Amides are slightly acidic, with a pKa around 15. When lithium aluminum hydride is introduced, the first step is deprotonation, forming a negatively charged species. However, just as in the case of carboxylic acids, lithium aluminum hydride still performs a nucleophilic attack on the carbonyl, generating a double negatively charged intermediate.
Again, aluminum coordinates with the oxygens, allowing one of them to act as a leaving group. But instead of losing the nitrogen, the oxygen is expelled, forming an imine-like intermediate. A second nucleophilic attack by lithium aluminum hydride then converts this intermediate into an amine. After acidic workup, the final product is a primary amine, not an alcohol.
If the starting material is a cyclic amide (a lactam), the mechanism follows the same pattern. However, unlike esters, lactams do not undergo ring opening. Instead, the carbonyl is completely removed, leaving behind a cyclic amine.
I challenge you to work through the mechanism for lactam reduction. It follows the same steps as the amide reduction, except there is no initial proton transfer because there are no acidic hydrogens available. If you follow the steps correctly, you should arrive at a cyclic amine as the final product.
Reduction of Nitriles
Finally, let’s discuss the reduction of nitriles.
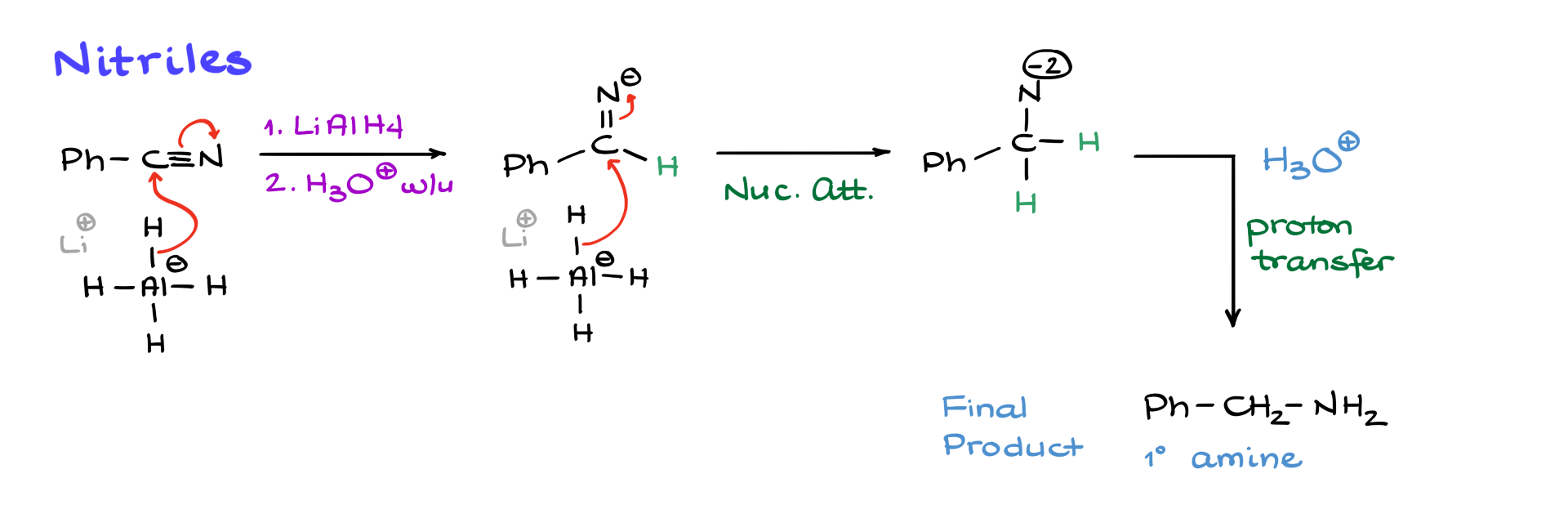
Although nitriles may not look like typical carboxylic acid derivatives, they are closely related. The carbon in a nitrile is electrophilic, though not as much as in other carboxylic acid derivatives. However, lithium aluminum hydride is such a strong nucleophile that it still reacts with nitriles.
The first nucleophilic attack by lithium aluminum hydride pushes electrons onto the nitrogen, forming a negatively charged intermediate. A second equivalent of lithium aluminum hydride then attacks again, generating a double negatively charged species. While the exact structure likely involves lithium and aluminum coordination, we don’t need to worry about that. Adding water and acid during workup leads to protonation, yielding a primary amine as the final product.
Recap
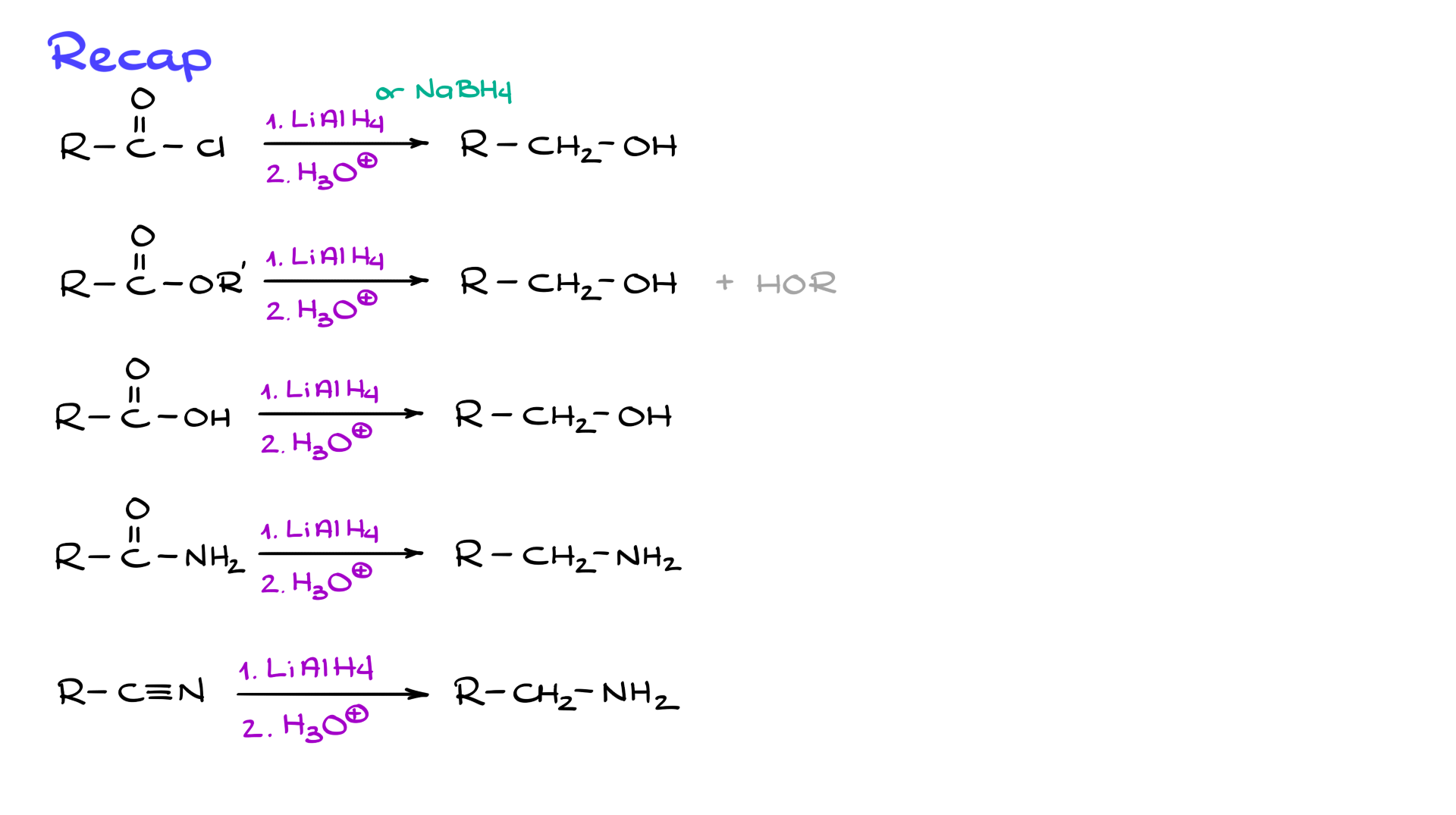
To summarize, when reducing carboxylic acid derivatives with lithium aluminum hydride:
• Acid chlorides and esters are reduced to primary alcohols.
• Carboxylic acids are also reduced to primary alcohols, though the reaction starts with deprotonation.
• Amides and nitriles are reduced to amines, not alcohols.
• Lactones open into diols, while lactams remain cyclic but lose the carbonyl.
While these reactions may seem numerous, they all follow the same fundamental mechanism with slight variations. Once you understand the core principles, predicting the products becomes much easier. Keep practicing, work through the mechanisms carefully, and soon enough, you’ll be able to anticipate the outcomes effortlessly. Let me know if you have any questions!
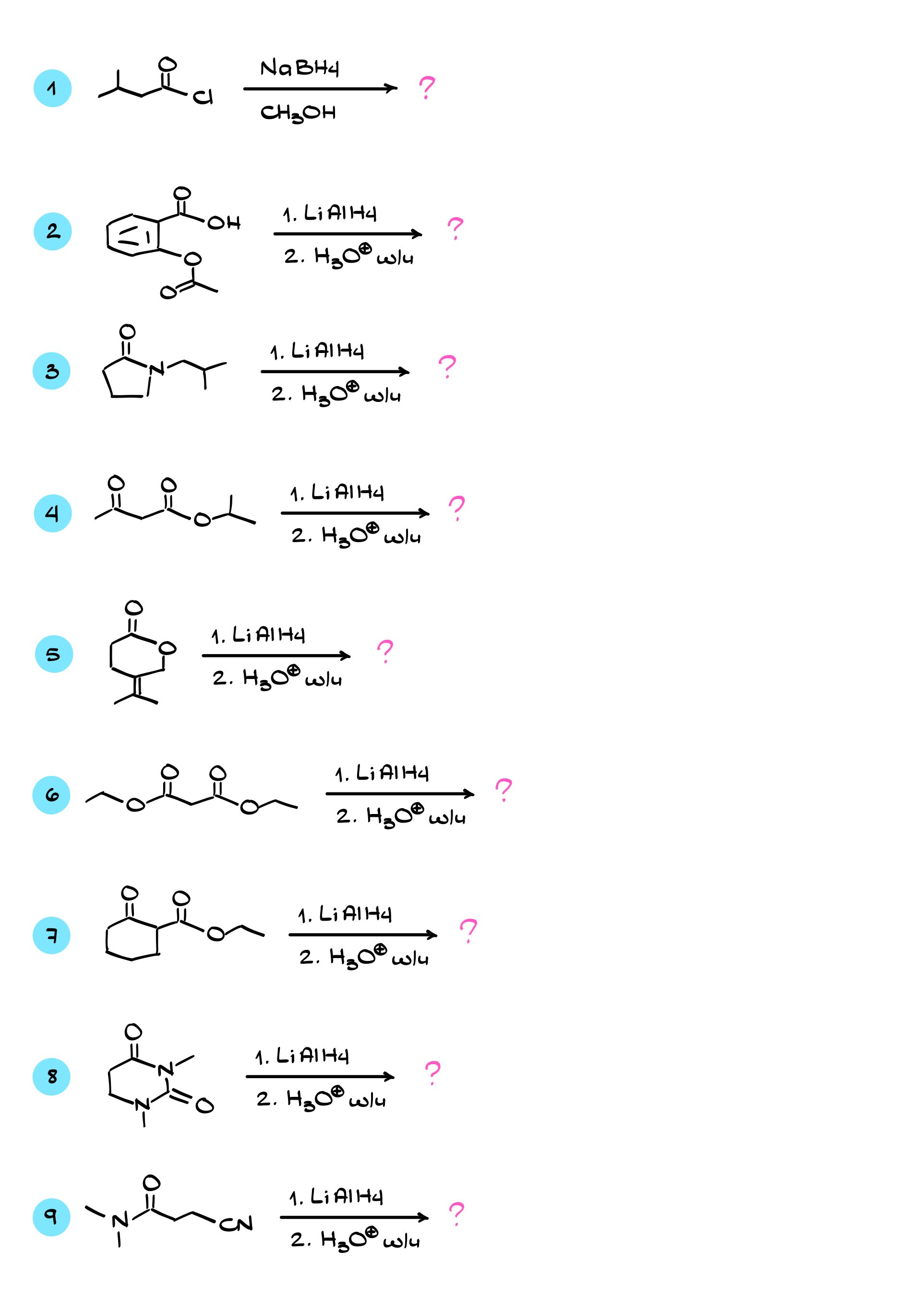
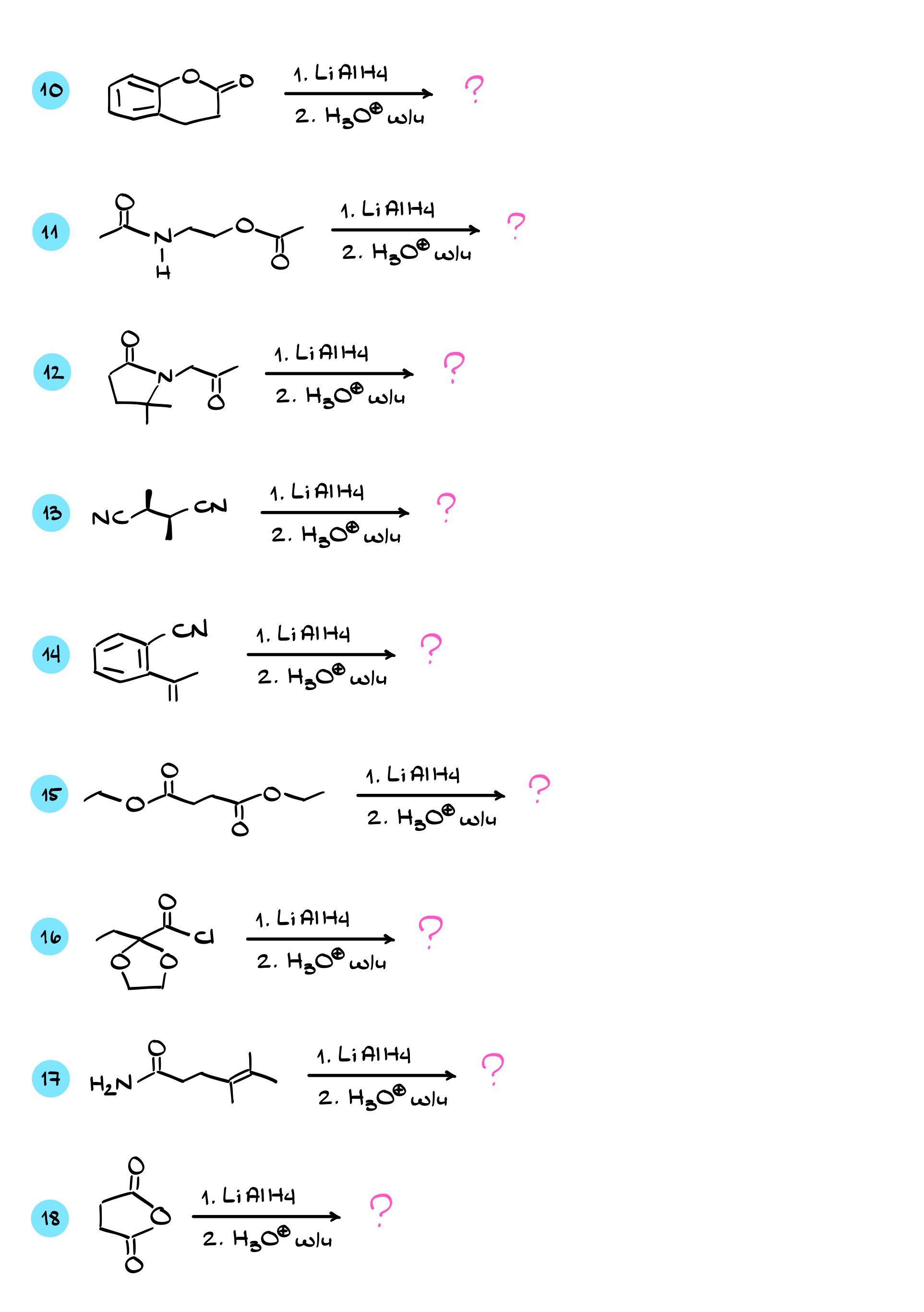
Practice Questions
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!