Reactions of Alkynide Ions
Alkynide ions are fascinating entities in the realm of organic chemistry, often referred to as “magic nucleophiles” for their remarkable capability to forge new carbon-carbon bonds. In this tutorial, we’ll dive deep into the world of alkynide ions. We’ll explore how to synthesize these nucleophiles and examine the spectrum of reactions they undergo. But here’s a word of caution: while alkynide ions might seem like a wonder solution for all your carbon-carbon bond formation challenges, they’re not without their quirks and limitations. In fact, these very limitations are favorites among exam setters, sneaking into test papers with the aim of tripping up unsuspecting students. But don’t fret; by the end of this guide, you’ll be well-equipped to tackle any alkynide ion curveball thrown your way!
Making the Alkynide Ions
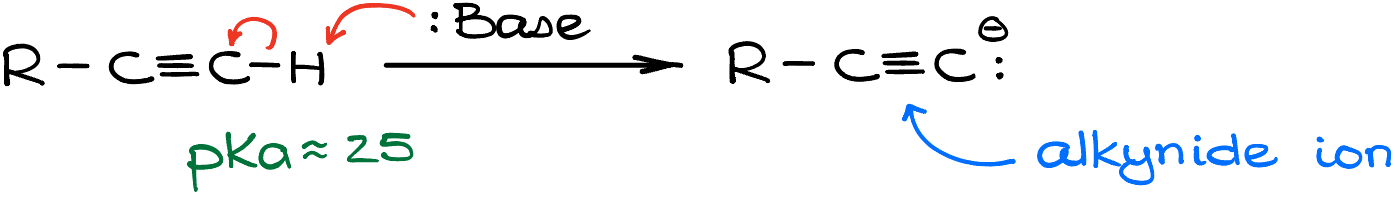
Before diving deep into the reactions of alkynide ions, let’s first unravel the mystery of their formation. The genesis of these ions begins with the reaction of a terminal alkyne and a strong base. This reaction results in the deprotonation of our substrate, essentially stripping it off its proton.
Choosing the Right Base
Here, a crucial question arises: What base should we opt for? Or, to frame it differently, how potent does our base need to be? To answer this, it’s essential to understand the acidity level of terminal alkynes. If we were to glance at a pKa table, the pKa values for terminal alkynes typically hover around the 25-26 mark. What this tells us is that terminal alkynes aren’t especially acidic. Consequently, to successfully deprotonate them, we’ll need a base that’s incredibly strong. An essential point to remember is that if our acid has a pKa value of 25, the pKa of its conjugate acid should exceed that number.
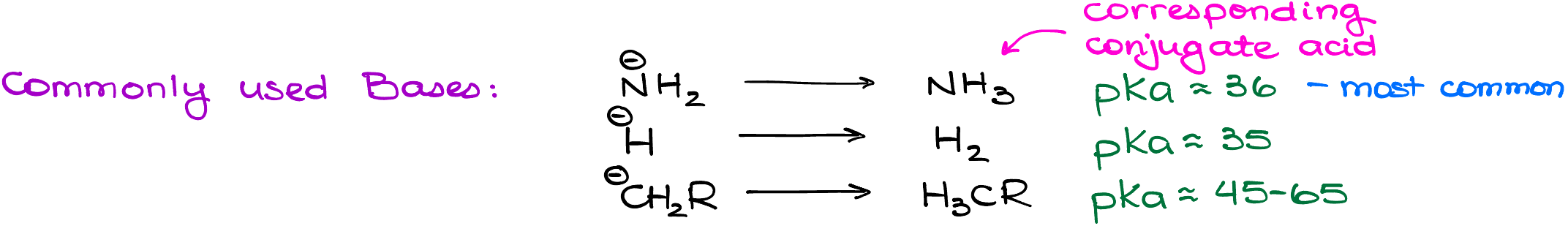
Popular Bases
Navigating through the myriad of bases, a few stand out in terms of popularity and efficiency – the amide, hydride, and alkyl ions. Among these, the amide ion takes the limelight, making frequent appearances in most organic chemistry courses.
An Important Note on Amides
As a small yet significant side note, an amide ion requires a counter ion to maintain charge balance. More often than not, the compound that’s prominently showcased in textbooks and lectures is sodium amide. This compound plays a pivotal role in the formation of alkynide ions, ensuring the reaction proceeds smoothly.
Real-World Example
Let’s break down this process with an example using tangible molecules to gain a better understanding. Consider the molecule propyne. When propyne is treated with sodium amide, an intriguing chemical dance takes place.

The Outcome
The end result of this reaction is the formation of the alkynide anion, accompanied by the production of ammonia. This is a classic example showcasing the prowess of sodium amide in plucking out that stubborn proton from the terminal alkyne.
Evaluating the Reaction’s Favorability
To ensure our confidence in this reaction and ascertain that it isn’t just a whimsical chemical dream, we can turn to the trusted method of estimating the equilibrium constant (Keq). This is achieved by comparing the pKa values of our acid and its corresponding conjugate acid, much like we’d do for any standard acid-base reaction.
The Numbers Game
Digging into the numbers, the pKa value for our alkyne (propyne) stands at around 25, while for ammonia, it’s approximately 36. Crunching these values, we arrive at an impressive Keq of roughly 1011. With an equilibrium constant of this magnitude, it’s evident that the reaction is highly favorable, showcasing the efficiency of sodium amide in forming alkynide ions.
The Many Faces of Alkynide Ions
Now that we have our alkynide anion ready, what’s its game plan? These ions are a force to be reckoned with! They’re robust nucleophiles and pack an equally potent punch as strong bases, making them eager to interact with a range of electrophiles.
Alkynide Ions vs. Primary Alkyl Halides
Your first encounter in class might be seeing these ions react with primary alkyl halides. This dance is primarily an SN2 reaction, but here’s where things get spicy! This reaction has a fine print – it’s exclusive to 1° alkyl halides. Why? Our alkynide ions belong to that elite club of nucleophiles which are also super basic.
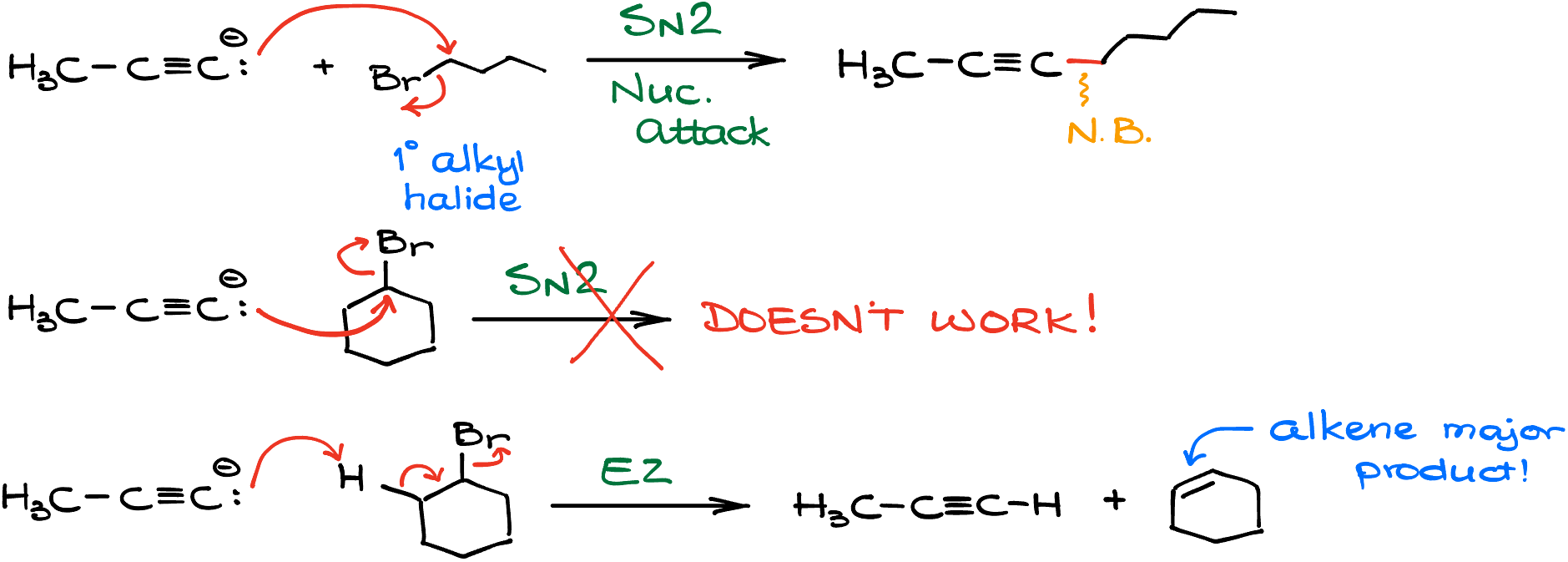
In the vast world of substitution and elimination reactions, these ions reserve their SN2 performance solely for primary alkyl halides. Venture into the territory of secondary alkyl halides, and you’ll witness a pivot to E2 reactions over SN2. So, if you’re hoping to see a substitution reaction here, you’re out of luck. What you will witness is the formation of an alkene through an elimination mechanism.
This subtle twist often ensnares students in exams like the ACS and MCAT. Why? Because not every maestro at the front of the classroom emphasizes this distinction. Heck, some might even gloss over this pivotal detail – a grievance I personally can’t overlook. So, here’s a pro-tip: when looking to form a new carbon-carbon bond using this reaction, stick to primary alkyl halides.
Beyond Alkyl Halides – Exploring Other Reactions
The plot thickens when you dive into different textbooks. Some might introduce you to the fascinating reaction between alkynide ions and epoxides, leading to the formation of alcohols.
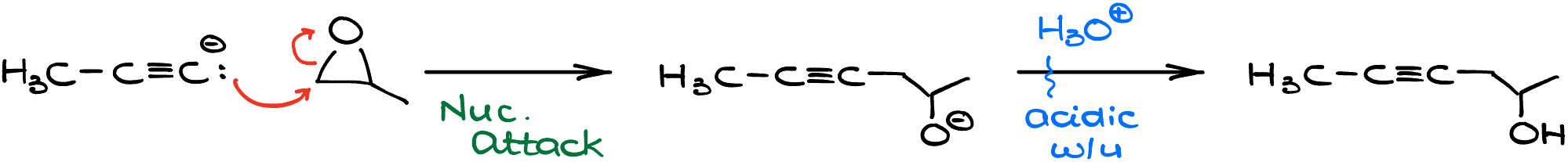
Here, the -OH group positions itself a carbon away from the alkyne. Another reaction to watch out for involves aldehydes and ketones. This pairing results in alcohols with the -OH group cozying up right next to the alkyne.
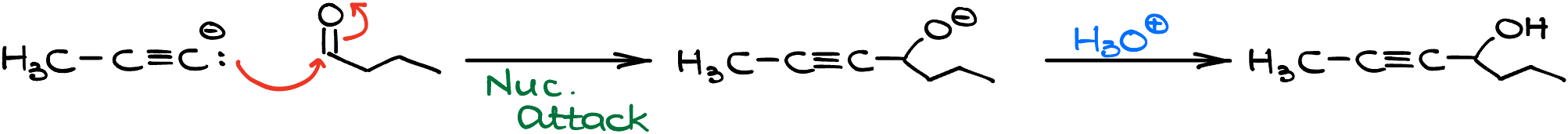
However, a heads-up! Unless you’ve got Clayden’s or Karty’s textbooks as your guide, you’re likely to primarily witness reactions with alkyl halides in this chapter. This is because the nitty-gritty of epoxides and carbonyls usually awaits you further down your organic chemistry journey.
From Humble Beginnings to Synthetic Maestro
You might wonder, how does the chemistry of alkynide ions fit into the grand scheme of things? Think of them as the unsung heroes in a multistep synthesis. Let’s dive into a captivating example.
Jazzing Up Ethylene
Starting with the most straightforward alkyne, ethylene, imagine it as a plain canvas waiting to be adorned with vibrant colors (or alkyl groups, in this case).
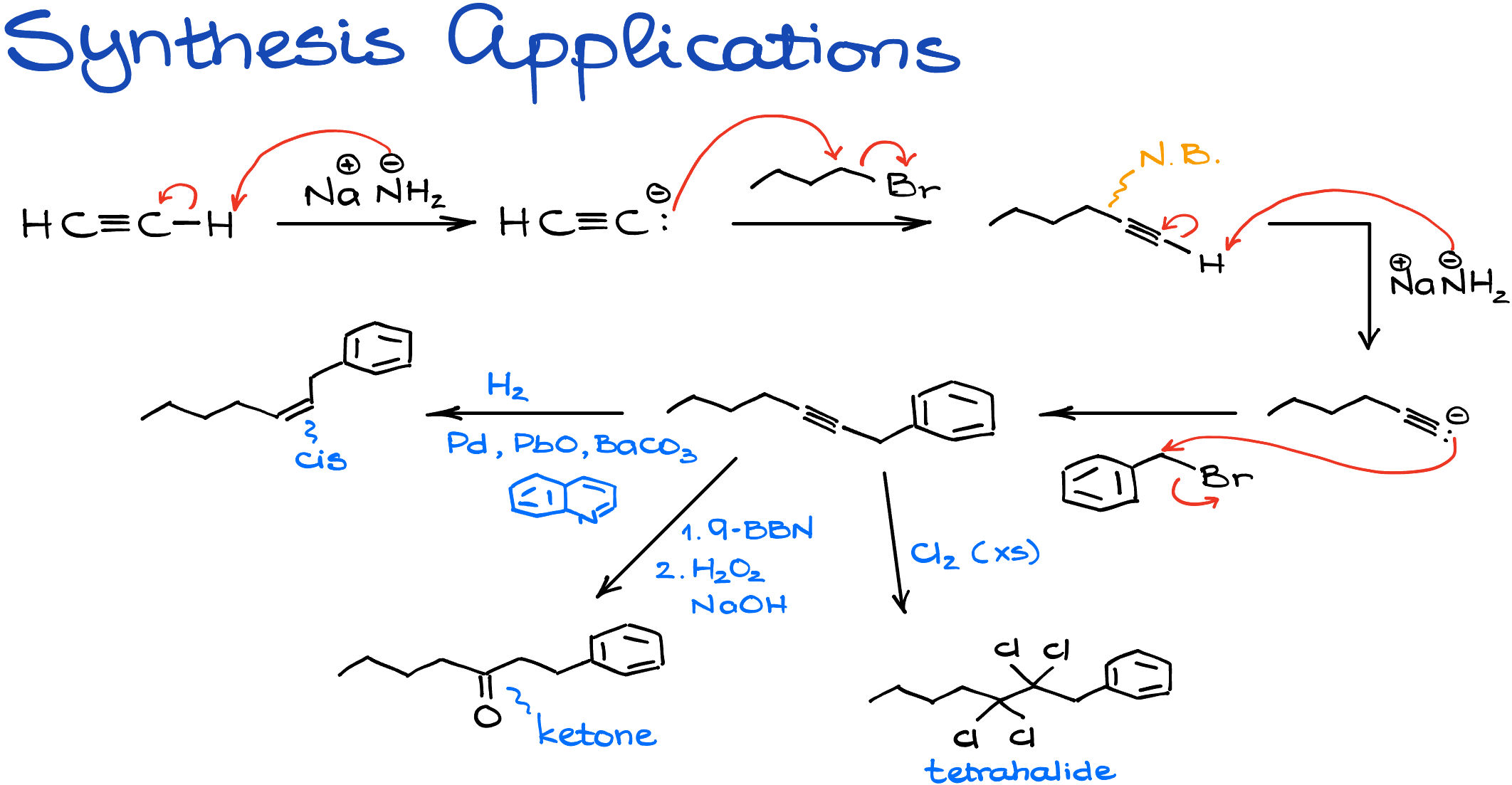
Step-by-Step Transformation
- Deprotonation: To kick things off, I’ll introduce ethylene to sodium amide, which promptly deprotonates it. Think of this as priming our canvas.
- Adding a Splash of Alkyl: With our primed ethylene, I’ll whisk in a primary alkyl halide, like butyl bromide. This step attaches an alkyl chain on one end, giving our molecule a bit more character.
- Round Two of Deprotonation: Not one to rest on our laurels, I’ll strip away another proton, prepping our molecule for its next transformation.
- Double the Alkyl Fun: With our freshly made alkynide anion, I’ll pair it up with another primary alkyl halide. The end result? A complex molecule boasting an alkyne functional group sitting proudly in the center. But that’s not the finale!
- Alkyne’s Many Faces: Given the remarkable versatility of alkynes, our synthesized molecule offers a treasure trove of possibilities. Whether it’s morphing into an alkene, transforming into a ketone, or even adopting a tetrahalide avatar, the options are vast.
Concluding Thoughts
Alkynide ions possess an exceptional ability to form new carbon-carbon bonds. Their synthesis is intriguing, starting with the reaction between a terminal alkyne and a potent base, most notably the amide ion. These ions aren’t just powerful nucleophiles but also display robust basicity. They’re choosy with their reactions, engaging in SN2 reactions primarily with 1° alkyl halides, while shifting to E2 reactions with secondary alkyl halides. Beyond alkyl halides, they can react with entities like epoxides and carbonyls, expanding their versatility. To truly appreciate alkynide ions, one can envision transforming simple ethylene into a multifaceted molecule with an alkyne core, opening doors to various subsequent reactions. Remember, while they may seem magical, a keen understanding is essential to avoid potential pitfalls in exams and real-world applications.
