Ranking Acids According to Their Strength without the pKa Table
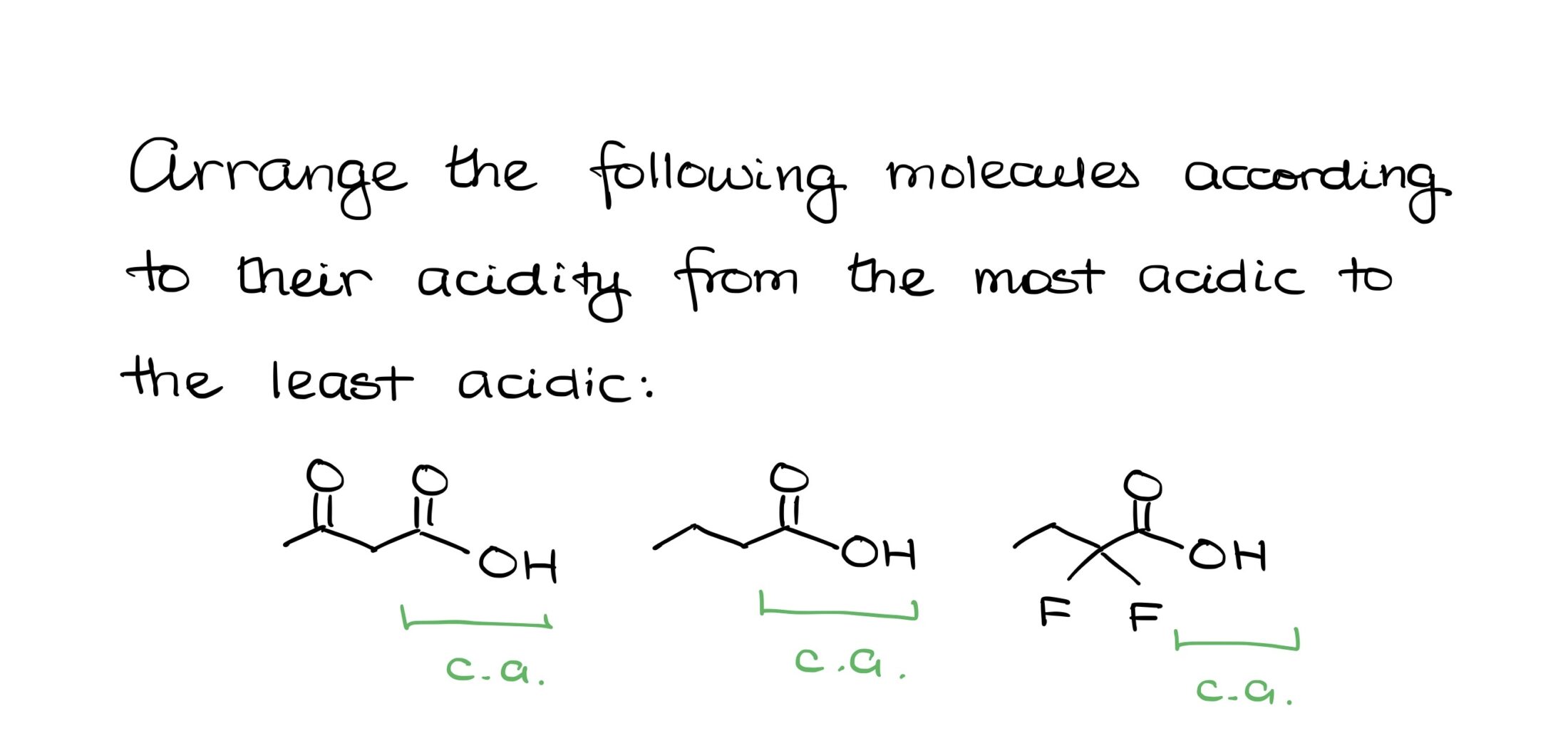
Suppose you’re sitting for an organic chemistry exam, and there’s a question asking you to arrange certain molecules in order of acidity. You reach for the pKa table, but there’s a catch: all the molecules are carboxylic acids, yet the table only provides one pKa value for the carboxylic acid group. So, how do you handle this?
Types of Acid-Base Ranking Equilibria
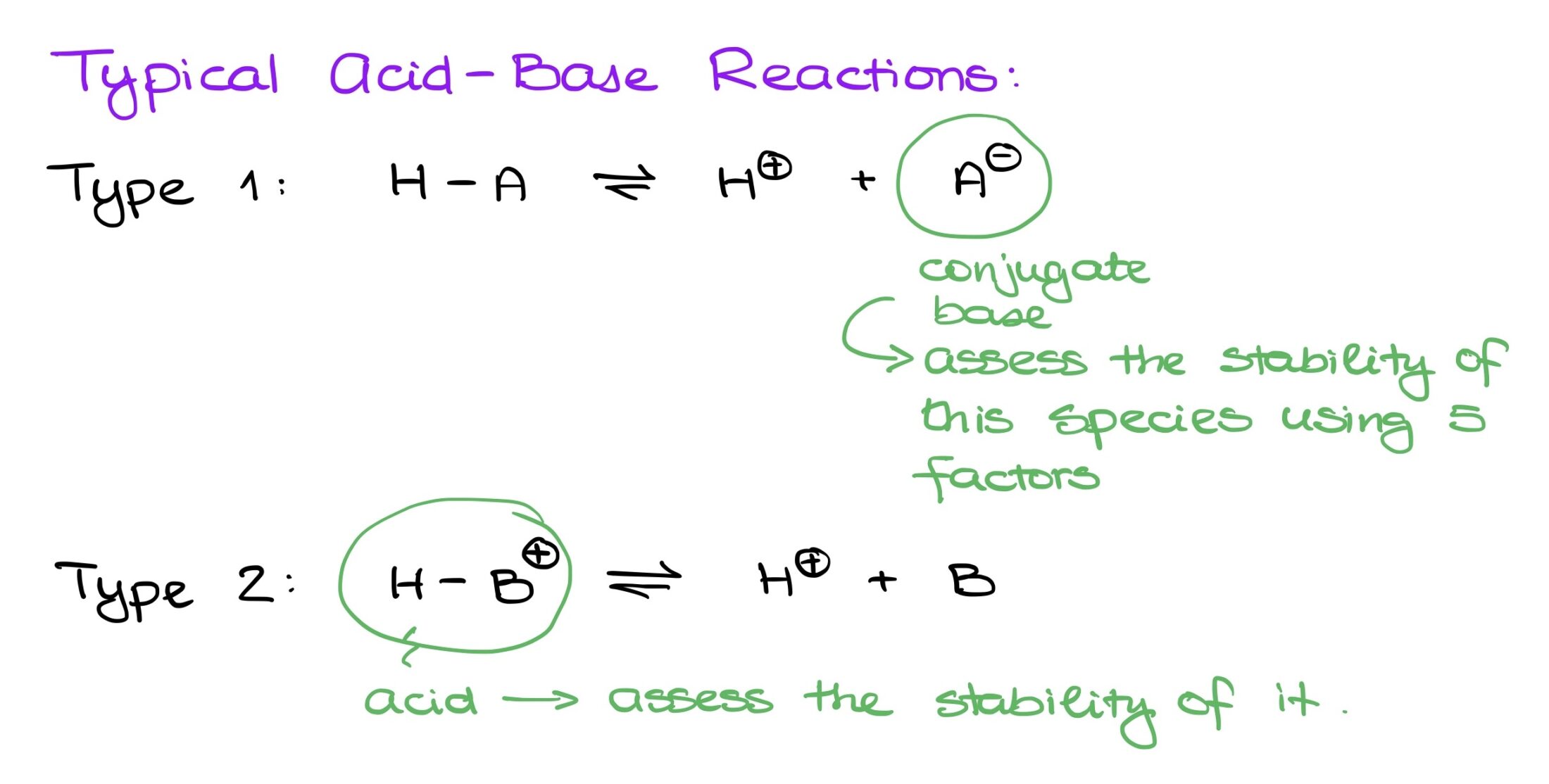
Let’s begin by understanding the basics of acid-base reactions. For simplicity, I’ll categorize them into two:
- Type 1: Here, an acid dissociates into H+ and its conjugate base (denoted as A-). In such reactions, we focus on the conjugate base’s structure, analyzing its stability using five key factors.
- Type 2: This involves reactions starting with a positively charged molecule (HB+), dissociating into H+ and a neutral conjugate base (B). In this type, we concentrate on the stability of the starting material, using the same five factors as in Type 1, but often in reverse.
For today, we’ll delve into Type 1, which you’ll encounter frequently in your studies.
Factors to Assess Stability of the Conjugate Bases When Ranking Acids’ Strength
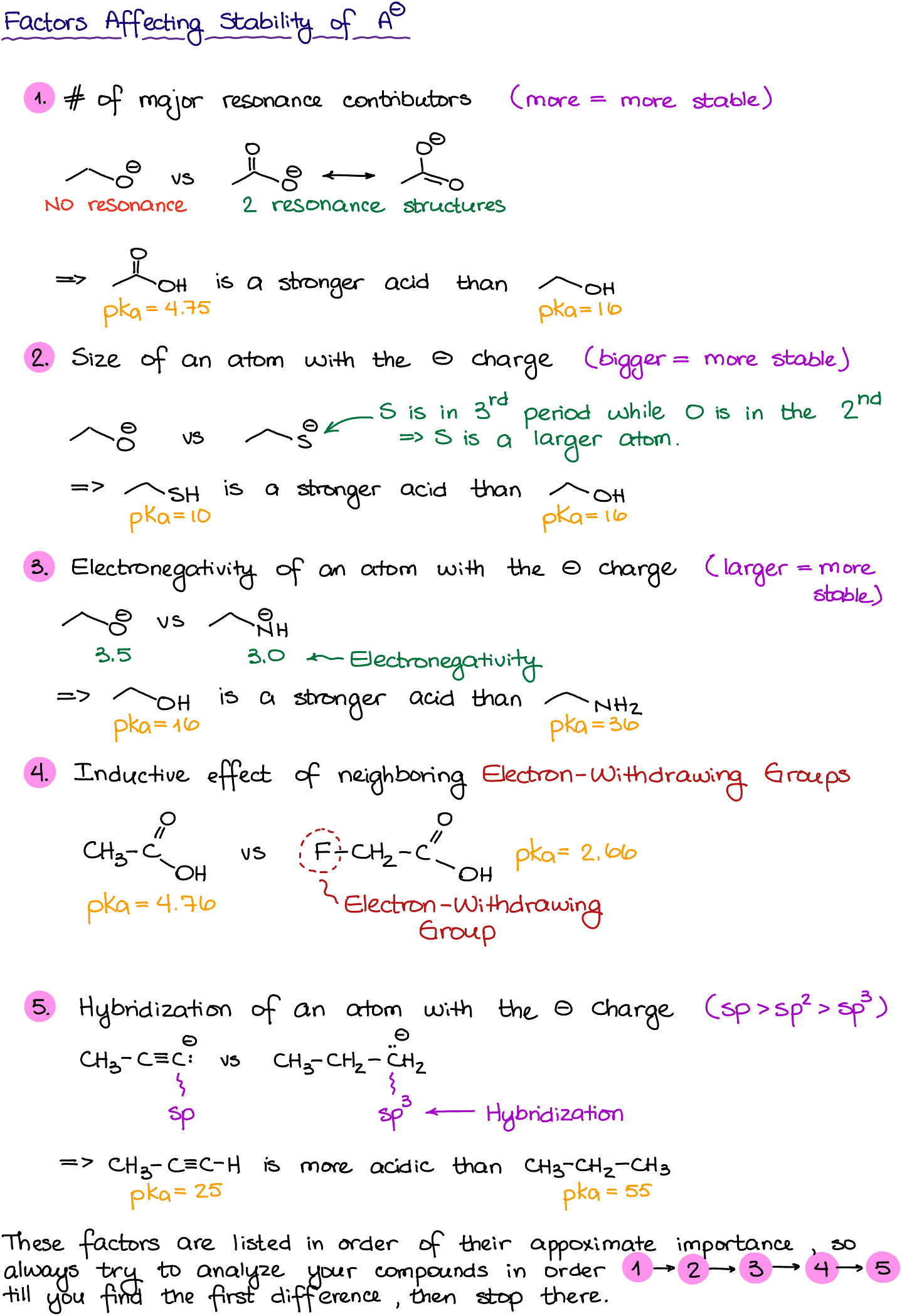
Here are the five factors determining the stability of our conjugate base:
- Resonance: Consider acetic acid versus ethanol. If we deprotonate them, we get acetate anion and alkoxide anion respectively. The acetate ion benefits from resonance, spreading the negative charge across two oxygen atoms, making it more stable.
- Atomic Size: Comparing an OH-containing molecule to an SH-containing one, sulfur, being larger than oxygen, stabilizes the negative charge better. Thus, molecules with SH are more acidic than those with OH.
- Electronegativity: Within the same period of the periodic table, the atom with higher electronegativity will better stabilize the negative charge. For instance, an alcohol (with oxygen) is more acidic than an amine (with nitrogen).
- Inductive Effect: Fluorine, a highly electronegative atom, can enhance the acidity of a carboxylic acid when attached, due to its electron-withdrawing effect.
- Hybridization: Negative charge on an sp hybridized atom is more stable than on sp2, which is more stable than on sp3.
Remember the acronym ARIO:
- Atom (considering atomic size and electronegativity)
- Resonance
- Induction
- Orbital (related to hybridization)
Now, are you up for some practice?
