Pinacol Rearrangement
Pinacol rearrangement is a specific elimination reaction that vicinal diols go through in acidic conditions. Unlike a typical E1 reaction that gives you an alkene, the pinacol rearrangement gives you an aldehyde or a ketone instead. Here’s the general scheme for the reaction:
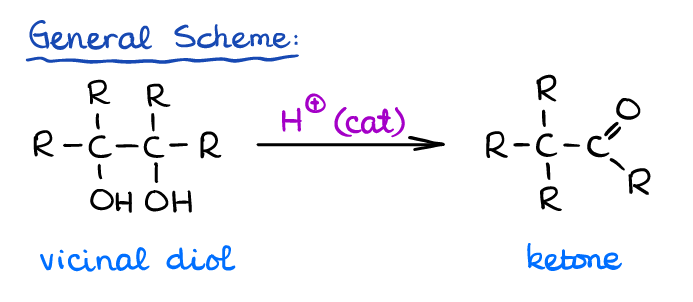
As you can see, the pinacol rearrangement also causes the carbon backbone change (hence, the rearrangement). This occurs via a carbocation intermediate.
Let’s look at the details of the mechanism of this reaction. It starts like any E1 of alcohols with the protonation of one of the hydroxyl groups (-OH) to form a leaving group (H2O).
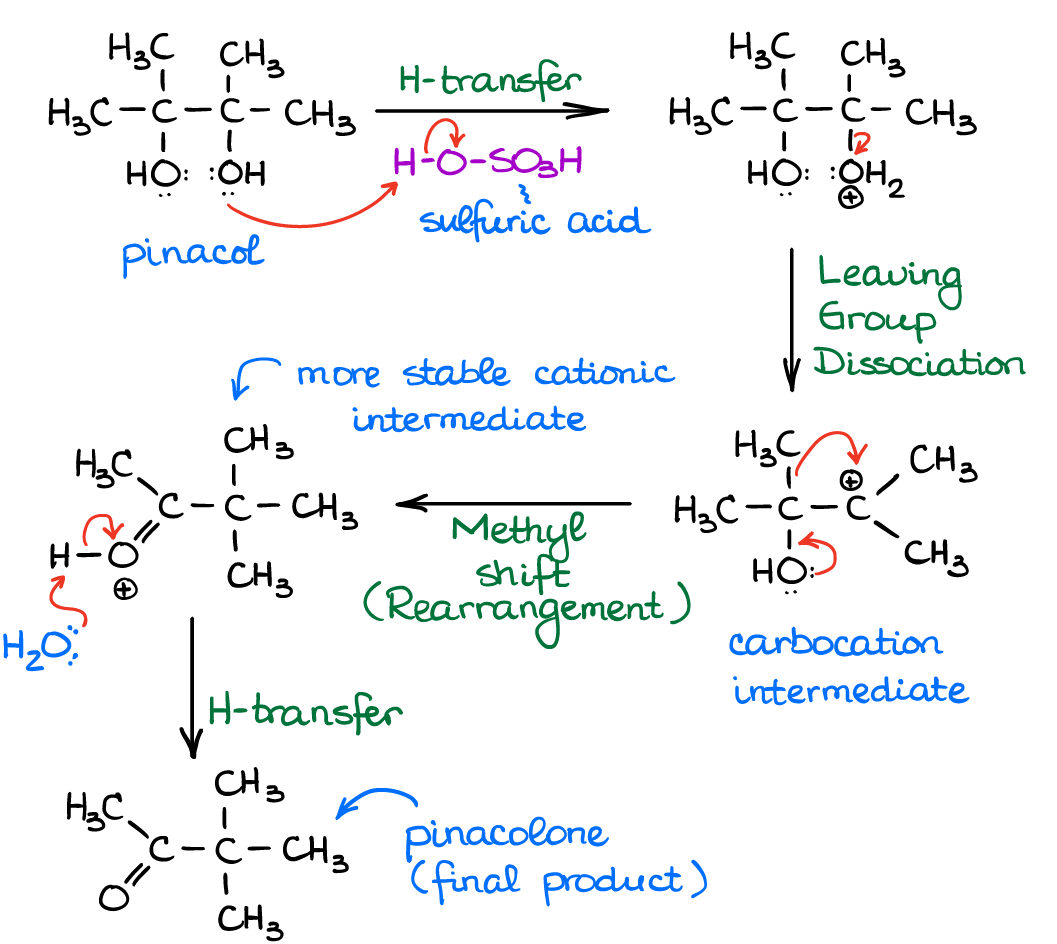
Once the leaving group dissociates to give you a carbocation, you’ll see that no matter how stable this carbocation is, it will tend to rearrange to give a new resonancely stabilized cationic species with the C=O double bond. We know that tertiary carbocations are comparatively stable, right? Well, when it comes to the C=O bond, it’s adds a ton of stability of the intermediate. I’m not gonna bore you with numbers here, just trust me on that. So long story short, the carbocationic rearrangement is driven by this additional stability.
BTW, you may also come across the name pinacolone rearrangement. This is because the name of the product of the pinacol rearrangement (with the actual pinacol as the starting material) is pinacolone. So the both names are kind of interchangeable.
Competing Groups in the Carbocation Rearrangement
So, what do we do when we have different groups competing for the same shift in the rearrangement? Just like in a typical carbocation rearrangement, we have certain migratory aptitudes.

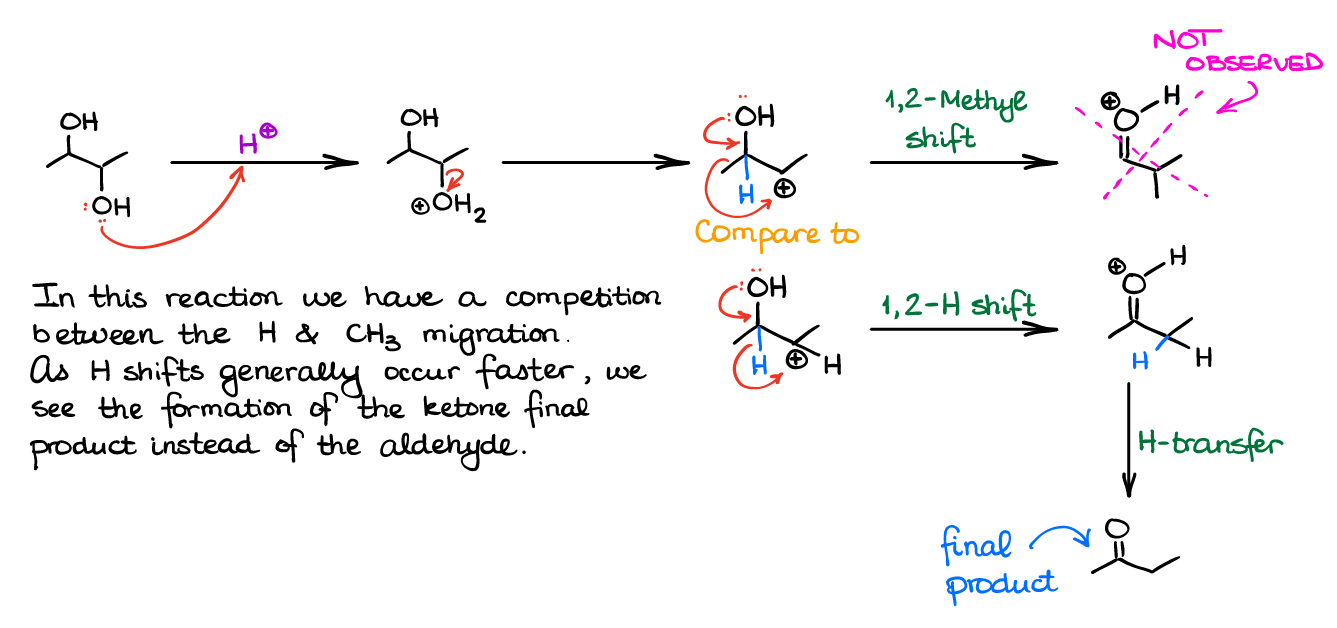
The pinacol rearrangement is not special in any way or form from any other rearrangement involving carbocations. So, our migratory aptitude stays the same: H migrates the easiest, then we have the Ph group, and then we have alkyl groups.
For instance, here’s the example when you have H and -CH3 competing in the rearrangement.
Likewise, here’s the example with the -Ph and -CH3 competition:
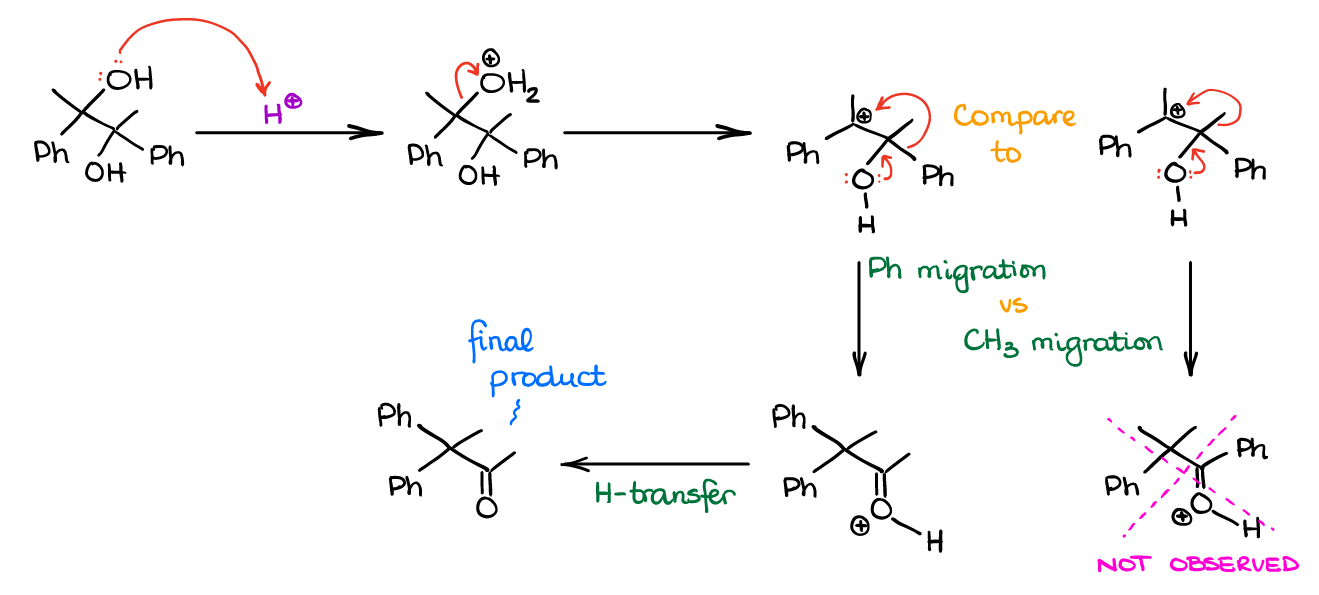
Notice, that while the product with a conjugated C=O and Ph (adjacent to each other) may be more thermodynamically stable at the end, this is still not the major product. When dealing with the pinacol rearrangement always remember the mechanism and the migratory ability and don’t pay attention to the stability of the final product.
Pinacol Rearrangement in Asymmetrical Diols
Alright, now when we know the details of the pinacol rearrangement mechanism, let’s look at more complicated cases. Fist, how to approach questions with asymmetrical diols? Consider the following:
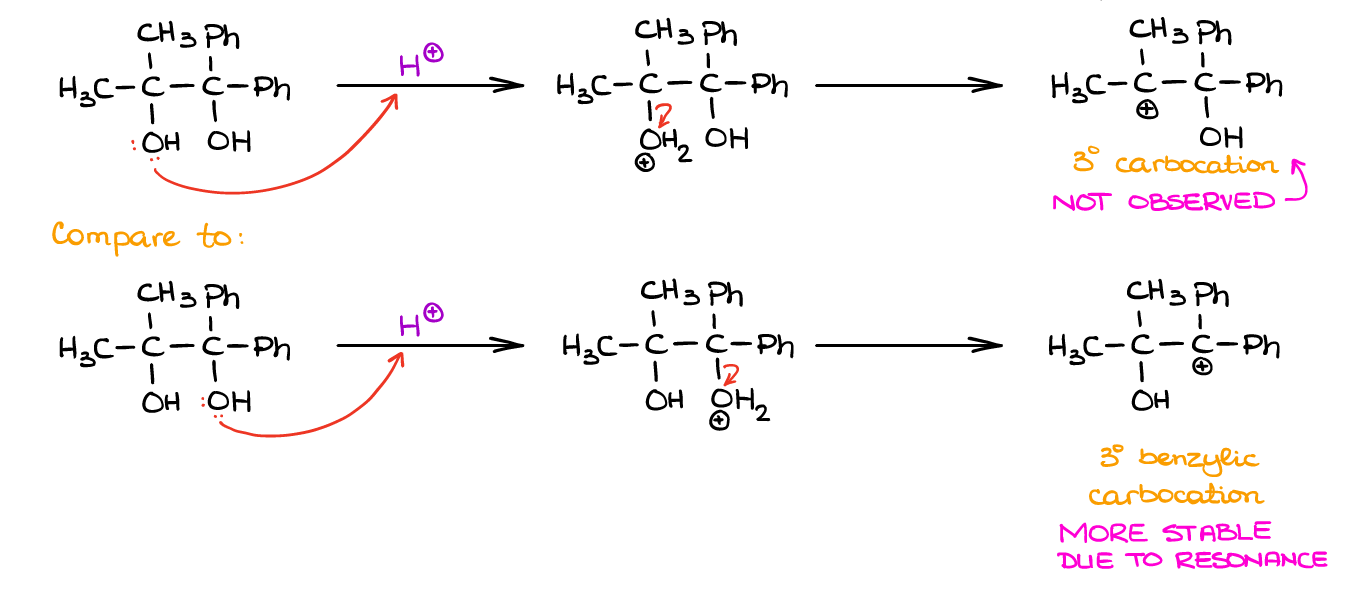
The trick is to consider the nature of the carbocation you’re going to get once the leaving group leaves. Since anything that can be protonated, will get protonated, it’s pointless to analyze the molecule from the perspective of the starting material itself. However, once you form the carbocation, you can see the differences.
In the example above, we have a typical tertiary (3°) carbocation vs the resonancely stabilized benzylic carbocation. Since the resonance stabilizes carbocations better than induction or hyperconjugation, the benzylic carbocation is way more stable than even the tertiary one. And since the more stable intermediates have lower activation energies leading to their formation, they form faster. So, while anything can be protonated in the solution, the H2O leaving group will dissociate easier from whatever carbon that can give you a more stable carbocation. Thus, the rest of the mechanism for the reaction above will look like this:
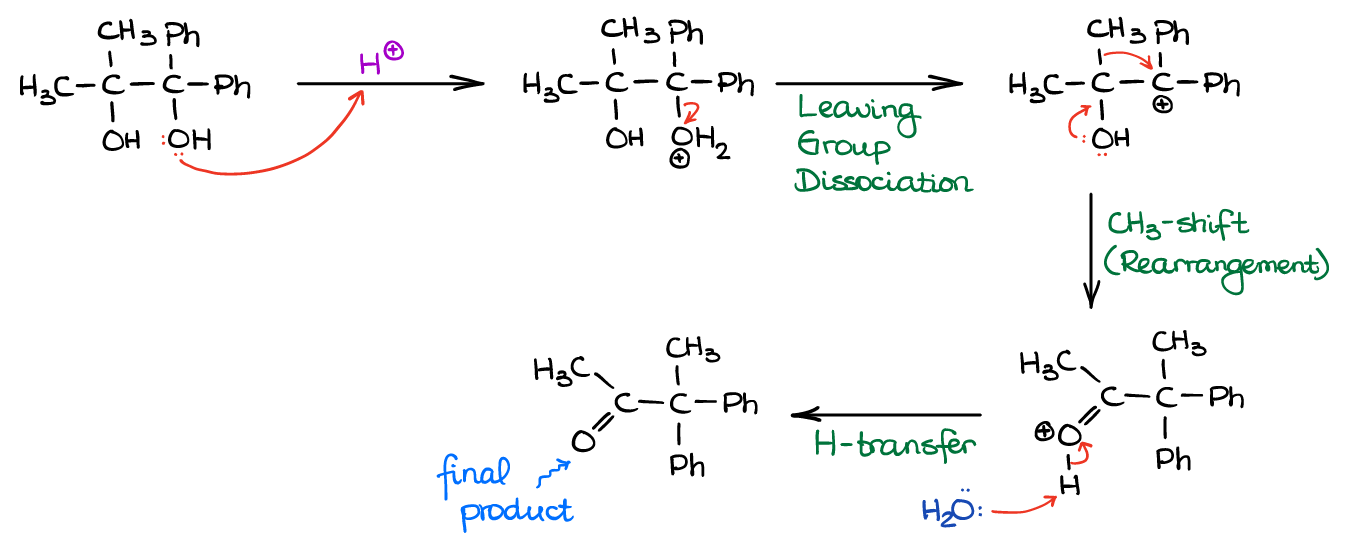
Again, although the product with C=O adjacent to Ph (resulting from the less stable carbocation) would be thermodynamically more stable, it doesn’t form.
Pinacol-Like Rearrangements of Epoxides
Some epoxides can open forming carbocations in acidic conditions. As these carbocations look exactly same as the carbocation in the pinacol rearrangement, they can undergo similar mechanistic pattern leading to the formation of a carbonyl compound.
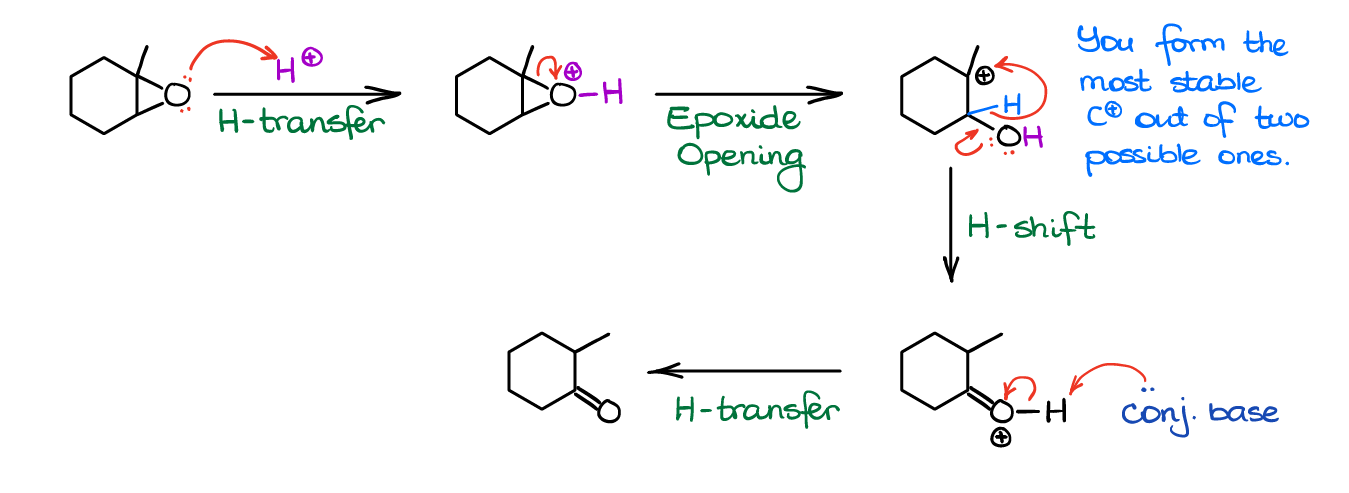
Note, only tertiary epoxides can go through this reaction. The secondary and primary epoxides do not normally form carbocations and will go through a typical epoxide opening via reaction with any available nucleophile.
