Oxidation of Alcohols with PCC
When we need to selectively oxidize our alcohol and stop at the formation of an aldehyde without over-oxidizing to a carboxylic acid, the first reagent that comes to mind is PCC.
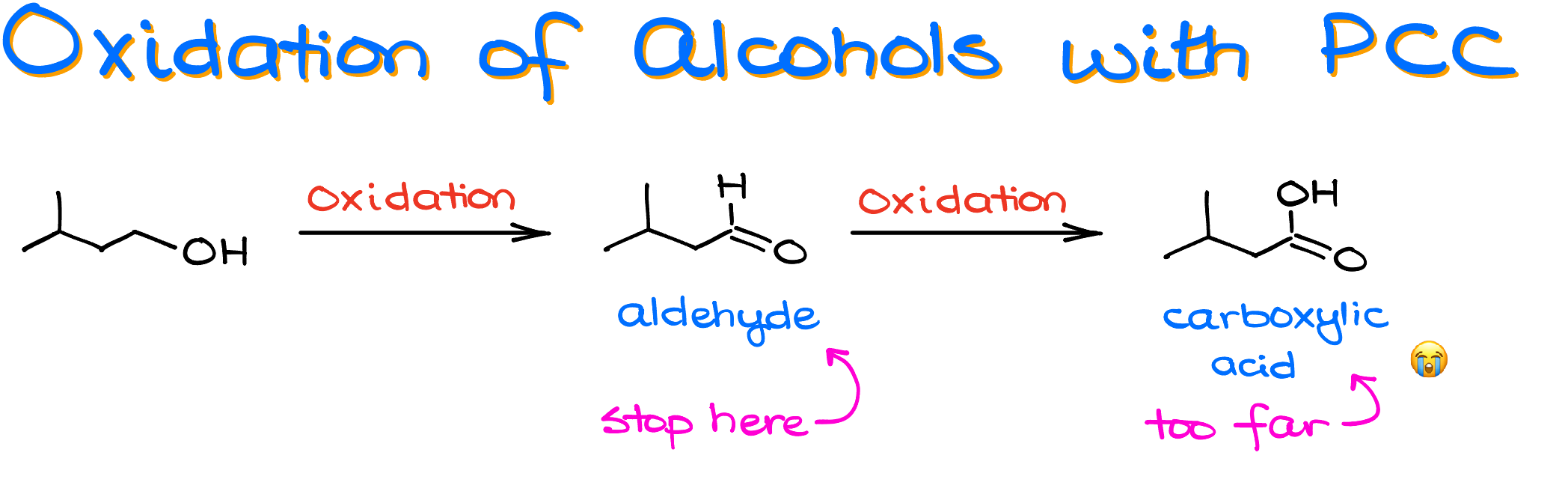
In the oxidation of alcohols and the Jones oxidation tutorials we’ve talked about the different methods how we can convert alcohols into aldehydes, ketones, and carboxylic acids. We’ve discussed that the oxidation in aqueous conditions promotes the over-oxidation of primary alcohols to carboxylic acids. So, the Jones oxidation won’t cut it when we need to stop at the formation of an aldehyde. But the problem is, the Jones reagent (chromium oxide in sulfuric acid) doesn’t dissolve in organic solvents well. Luckily, there are workarounds.
One of the most common alternative reagents that can be used in non-aqueous conditions is PCC, which stands for pyridinium chlorochromate. You may also come across the PDC (pyridinium dichromate) and the Collins reagent (chromium oxide in pyridine). And while there are some subtle differences in the reactivity of those oxidizing agents, we’ll treat them as interchangeable. For the rest of this tutorial, I’ll be using PCC.
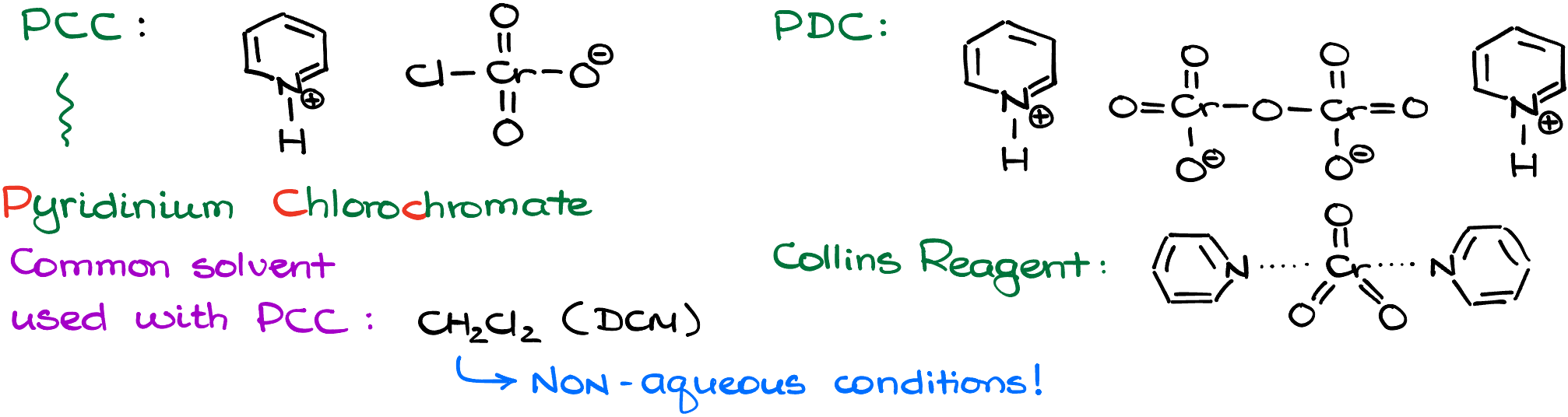
The important property of PCC and similar oxidizing reagents is that it dissolves in organic solvents. The most common solvent we’re going to see is DCM (dichloromethane). And since we can easily produce anhydrous (aka, without water) organic solvents, we can perform our oxidation reactions in the absence of water eliminating the threat of the overoxidation of primary alcohols to carboxylic acids.
Oxidation with PCC is also historically important. It was first described by Elias J. Corey and his student J. W. Suggs in 1975 and revolutionized the field of organic chemistry as the controlled oxidation of alcohols was very much needed at the time. And if you look at the research papers at the time, there’s a whole bunch of those coming up: PCC 1975, Swern oxidation early 1970’s, Dess-Martin oxidation 1983, etc. Nowadays, PCC fall out of favor though due to the chromium toxicity and the environmental and health-related concerns associate with the use of DCM.
Nonetheless, this is a classic and important oxidation reaction of alcohols, so you’ll be responsible for it on the test. So, let’s look at its mechanism.
Mechanism of the Oxidation with PCC
Oxidation of alcohols with PCC starts with a nucleophilic attack by the oxygen of the alcohol onto the chromium oxide. The protonated intermediate that we get after that attack will lose the proton to the pyridine which is going to be our base in this reaction.
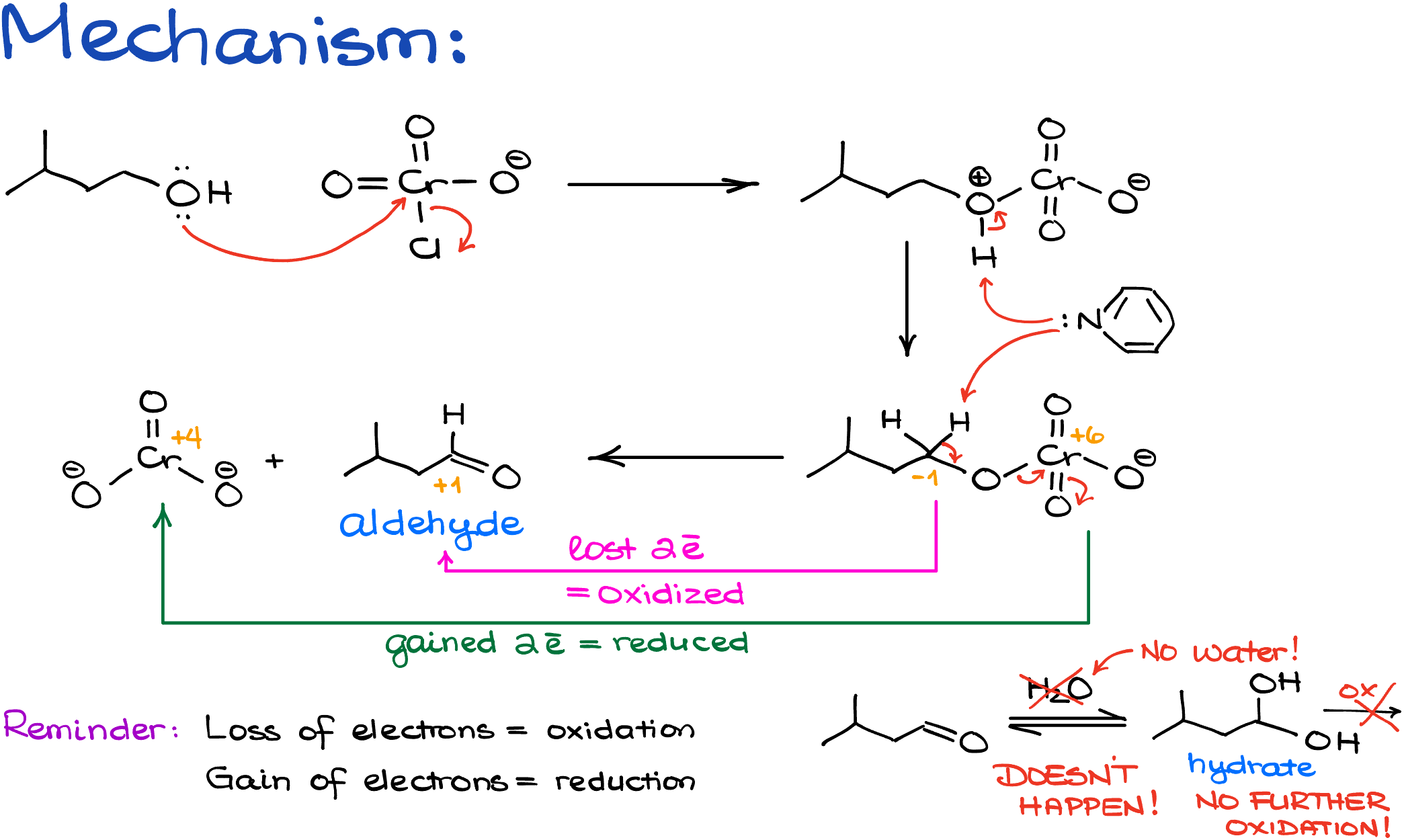
Next, pyridine will pull off one of the alpha hydrogens to create the C=O double bond. This is the red-ox step since the oxidation state of carbon changes from -1 to +1, and the oxidation state of chromium goes from +6 to +4. So, in other words, carbon is oxidized, and chromium is reduced. And since we never care about what happens to the inorganic co-products, we classify this reaction as an oxidation since carbon formally lost the electrons. And we know that the loss of electrons is oxidation.
Now, the important thing to remember here is that due to the absence of water, the aldehyde intermediate in this reaction cannot be converted into a corresponding hydrate. And if we don’t have an -OH, we cannot proceed with the second round of oxidation like we did in the case of the Jones oxidation. Thus, our molecule stays as an aldehyde.
So, essentially, what we’re doing here is the same mechanism as for the Jones oxidation. However, due to the anhydrous environment, we’ll never go into the second round of oxidation because our carbonyl can’t make a hydrate.
Examples of Oxidation of Alcohols Using the PCC
In the first example, I have a simple primary (1°) alcohol, butane-1-ol. Since the oxidation will stop at the formation of an aldehyde, I’ll end up forming the corresponding aldehyde, butanal.
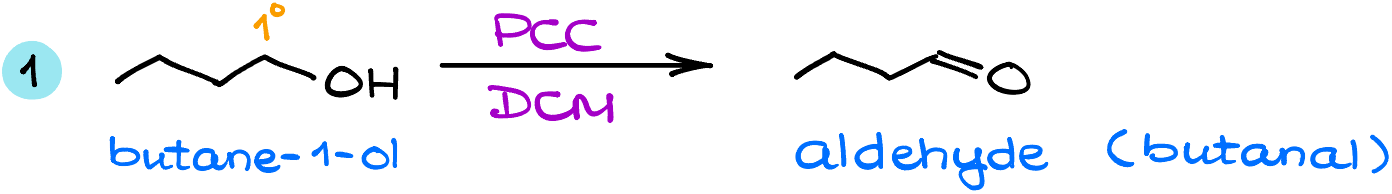
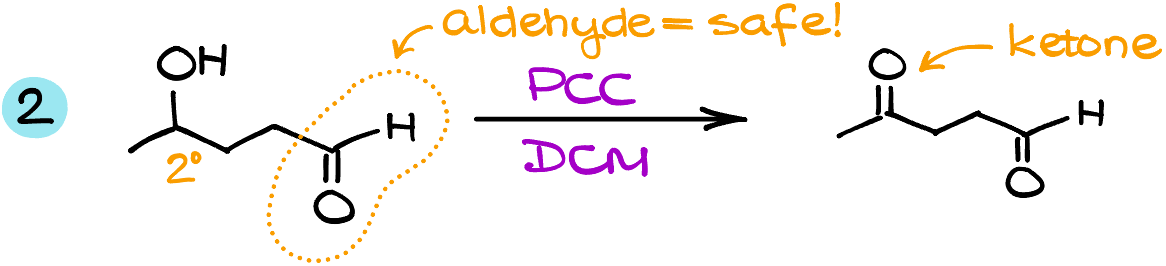
In the second example, I have a secondary alcohol and an aldehyde. Unlike the Jones oxidation, the reaction with PCC won’t do anything to the aldehyde, so it’s safe. The secondary alcohol, however, will turn into a corresponding ketone.
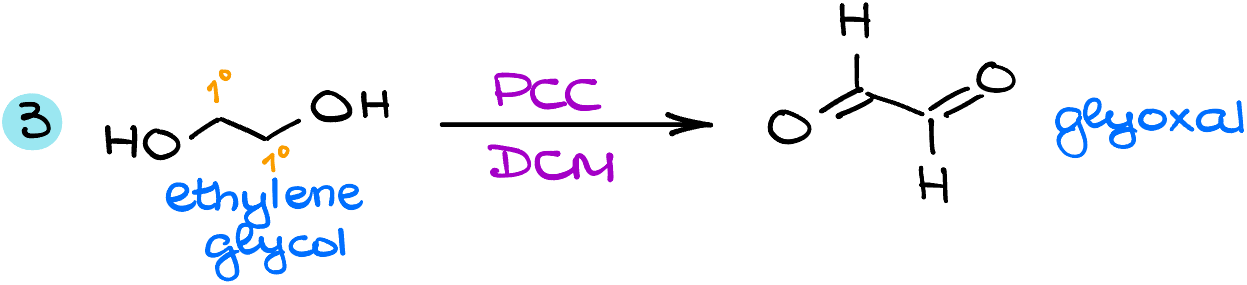
In the next example, I have ethylene glycol, an alcohol with two primary alcohol groups. The reaction with PCC will convert both of them into the corresponding dialdehyde, glyoxal.
And finally, in my last example here, I have a tertiary (3°) and a primary (1°) alcohol in the same molecule. Since the tertiary alcohols cannot be oxidized any further, we’ll leave it as is. The primary alcohol functional group, however, will give us the corresponding aldehyde.
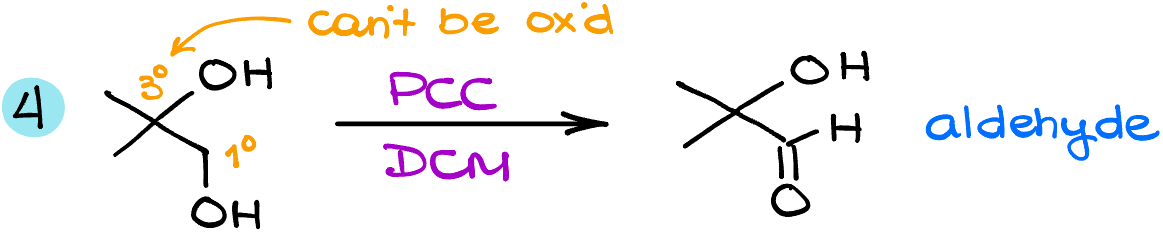
Concluding Thoughts
The PCC is an excellent oxidizing agent with a well-documented broad applicability and selectivity. Its main drawback is the chromium toxicity and some workup issues you’ll run into when performing the reaction in the lab. However, it’s an overall excellent choice of a reagent for you when you’re planning your synthesis in your organic chemistry class or brainstorming ideas for the reactions on the test.
