Newman Projections
When atoms in organic molecules connect via single bonds, you can have a free rotation around those single bonds. That gives you a different 3D forms of the same molecule. We refer to those forms as conformations of the molecules. These molecular conformations may be somewhat challenging to represent. For instance, let’s look at various representations of a pentane molecule:
If we look at the molecules above, they may seem a little chaotic. So, we need a somewhat better way of describing these conformations. But before we go into the nitty-gritty details of the conformations of the more complex molecules, let’s look at something very simple. The simplest molecule with a C-C single bond is ethane. Here are a couple of ways how we can represent ethane:
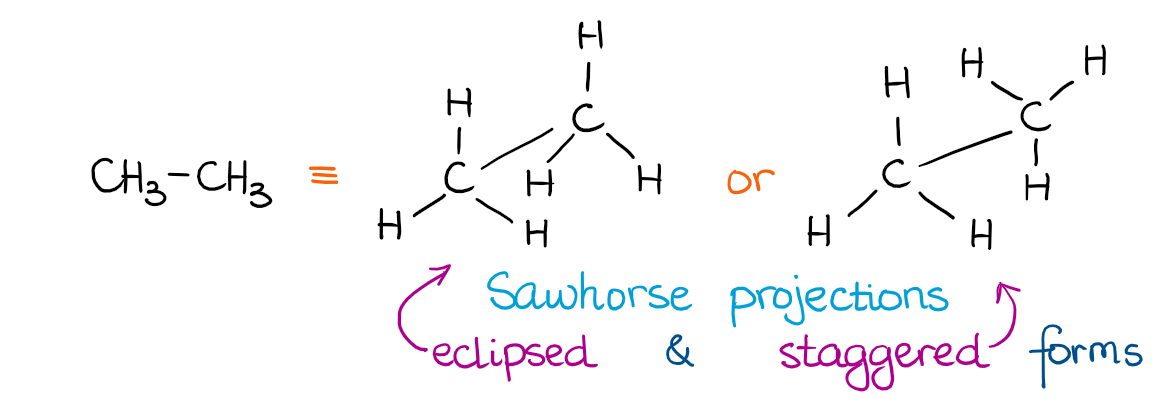
The two orientations of the CH3 group give us the two different orientations of ethane in space. We call those two conformations the eclipsed and staggered conformations. Here’s how it looks using a molecular model kit:
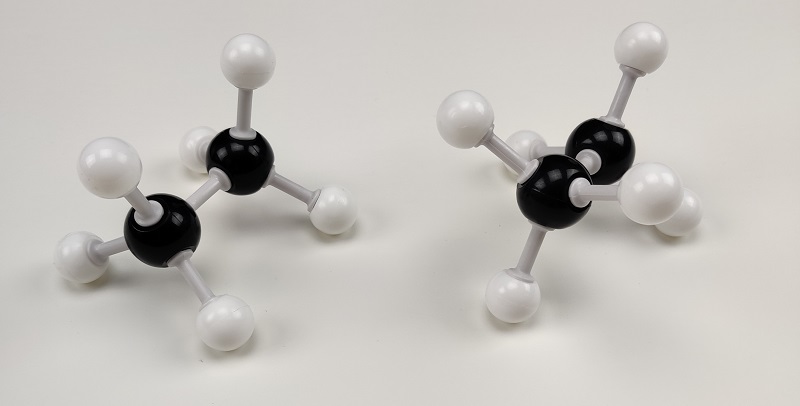
In the eclipsed conformation the H’s are directly behind each other. They are just like the Moon and the Sun during the solar eclipse. Thus the name eclipsed. 😹 In the staggered conformation, the atoms are in-between each other when we look at them directly. The dihedral bond angle in the staggered conformation is 60° while in the eclipsed it’s 0°.
What Are The Newman Projections?
The sawhorse projection I used above to illustrate the conformations is not a very convenient way of showing conformations. So, the more common way of representing conformations is the Newman Projections. The Newman projection of a molecule is a superimposition of the two atoms on a single bond showing dihedral angles between the groups on the front and the back atom.
In other words, I can show my sawhorse projections from above in the Newman projection form as such:
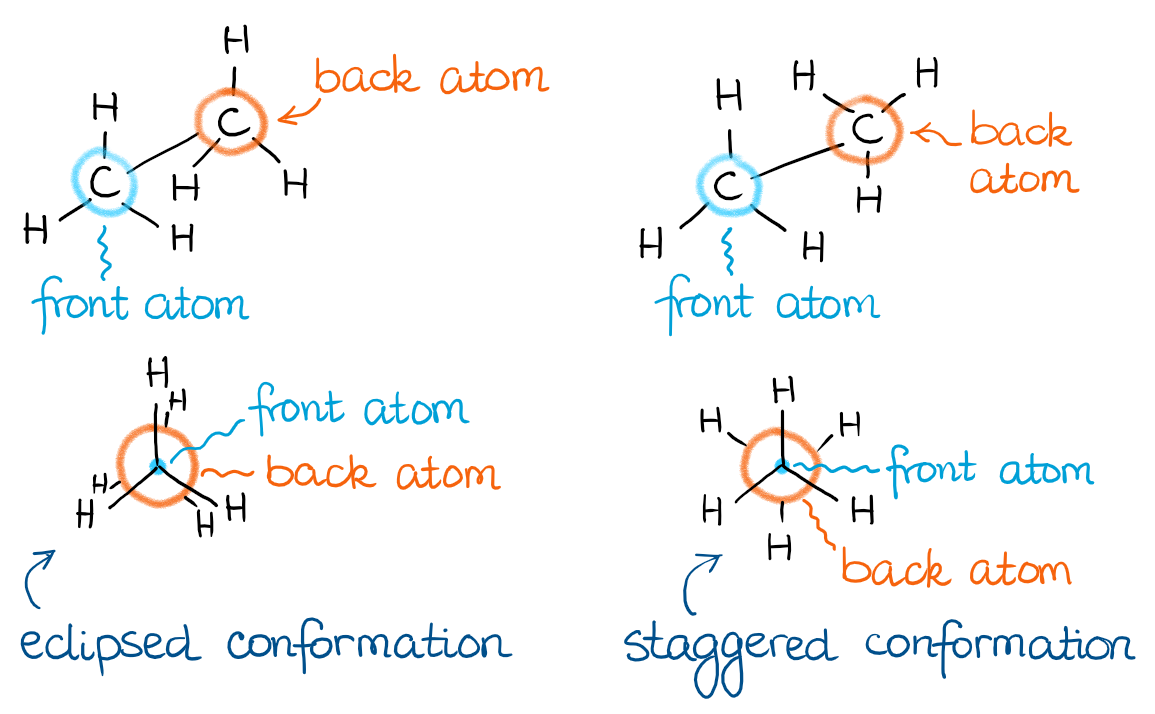
The direction from which you’re looking at your molecule is important for the construction of the Newman projections. Thus, you always need to indicate which atom is the front one and which atom is at the back. Traditionally, the front atom of the Newman projection is a dot, while the back one is a circle. When you’re drawing the bonds from each of your atoms, make sure you’re doing it very carefully to show exactly which atom those groups are sitting on. Remember, if your instructor has to guess, they’ll just take points!
Stability of Conformations
Staggered and eclipsed conformations have different stability due to the different distance between the groups on the adjacent atoms. The eclipsed conformation has a lower distance between the eclipsing atoms, therefore, it has a higher energy. As you know, the higher energy means lower stability. Contrariwise, the staggered conformations have a larger distance between the groups, and thus are more stable (lower in energy).
Thus, if I imagine the free rotation of the groups along the single bond in ethane, I’ll see the following energy plot:
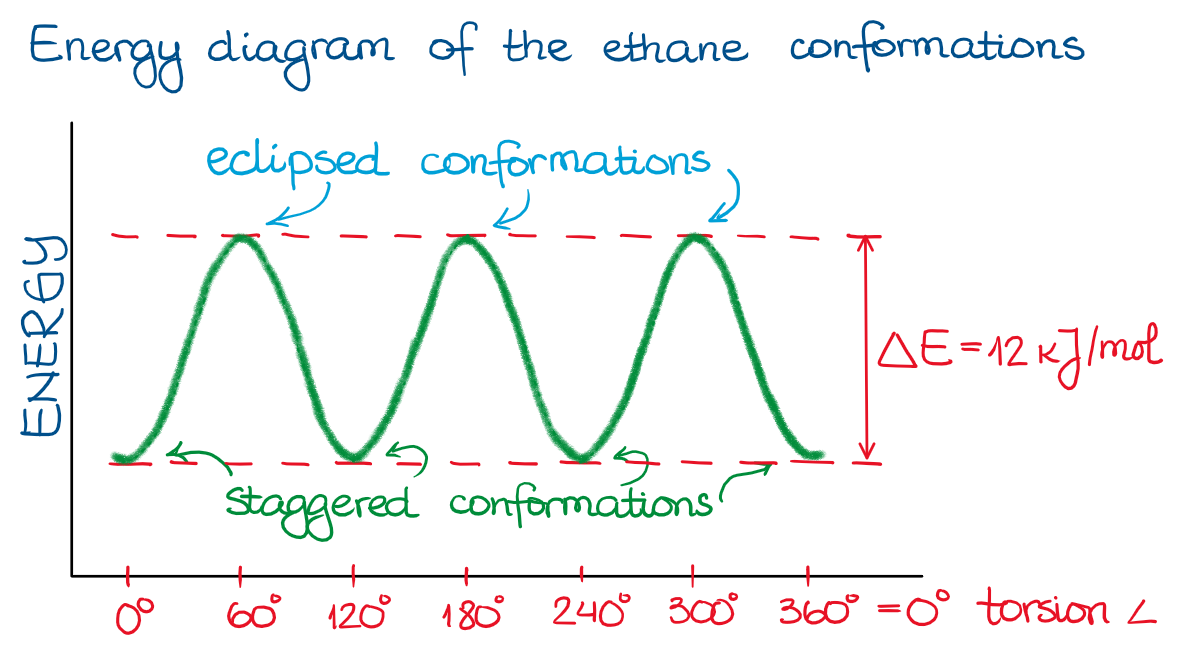
Since in the ethane example all the groups are the same (H’s), the energy profile is a simple up-and-down plot. For ethane, the energy difference between the staggered and eclipsed conformations are about 12 kJ/mol. While this is not too much (a typical bond strength is in hundreds of kJ/mol range), this energy is still sufficient to be detectable.
Now, let’s imagine we have something a little more complex. Something like butane, for instance, already has 4 carbons making the rotation around the single bond between carbons 2 and 3 more complex than in ethane. The energies of the different groups in staggered and eclipsed conformations for butane are:

Using this data, we can construct an energy profile of butane rotation around the bond between carbons 2 and 3:
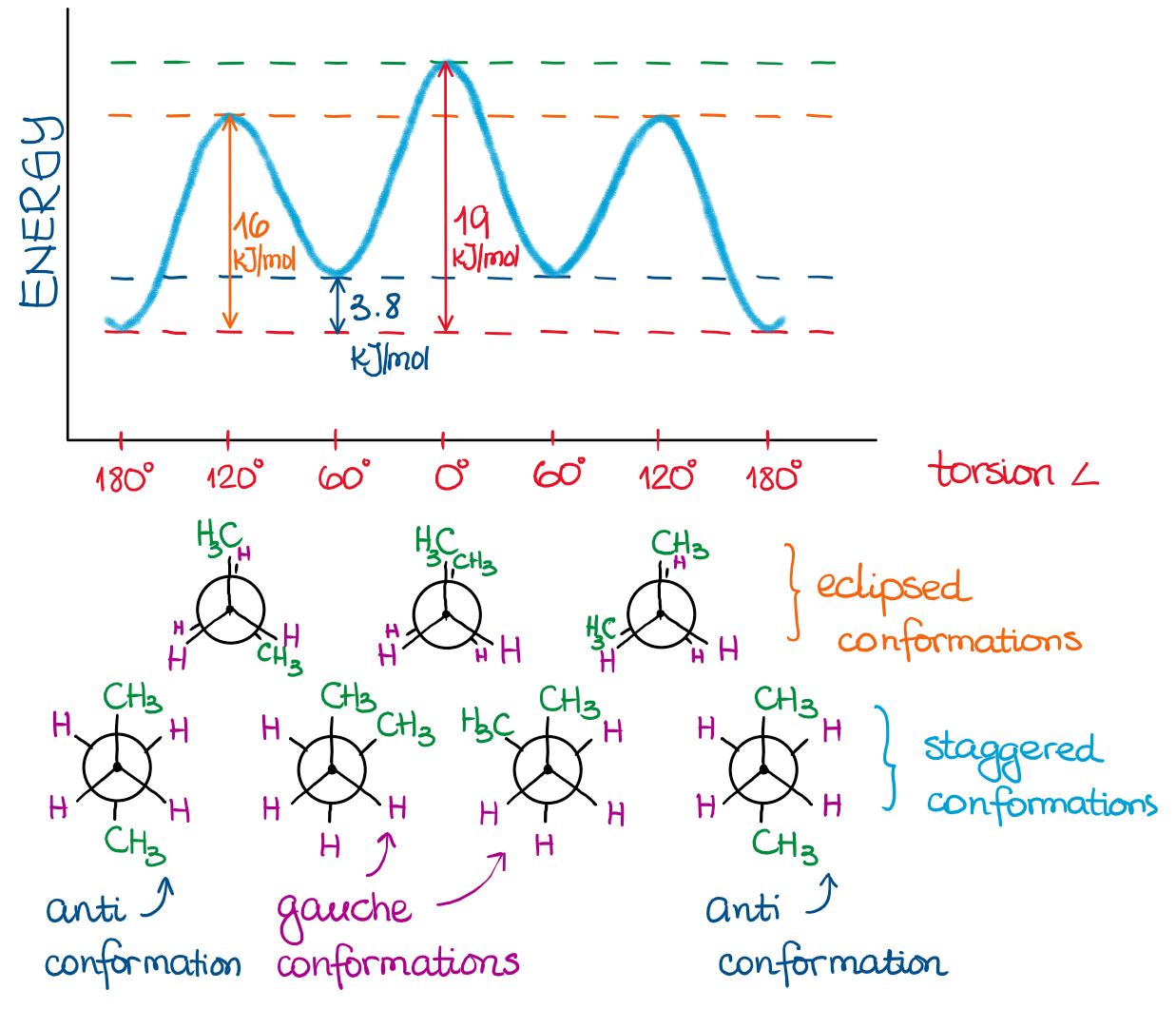
Since the methyl groups (CH3) are much larger than simple hydrogens (H), we no longer have degenerate energies for our conformations.
Names of Conformations
We also have special names for different conformations when the groups are no longer identical.
- When the two largest groups are directly behind each other, we call this conformation a total eclipse. This conformation has the highest energy. It’s the least stable.
- When the two largest groups are staggered and are at 60°, we call this conformation gauche from the french word meaning “to the left.”
- When the two largest groups are directly opposite to each other, we call it an anti conformation. This is the most stable conformation, thus it has the lowest energy.
I added the conformation drawings and names above in the energy diagram for your reference.
