Molecular Orbital Description of the π-Bond
Within the scope of a typical sophomore organic chemistry course, we are going to make one very important assumption: the σ-bonds and the π-bonds are independent of each other. This means that no matter what we do with one type of a bond, it won’t affect the other. Why is that important? Well, the short answer is—to make our life easier. While the σ-bonds and the π-bonds are not actually 💯 independent of each other and do have some limited interaction, this simplification works for our purposes. This approach to Molecular Orbital Theory (MOT) is called the Frontier Molecular Orbital Theory (FMOT).
How the π-Bond is “Constructed”
We get the π-bond when we have an interaction of two p-orbitals. As you remember from general chemistry, the p-orbital has a node and two lobes. Thus, when two p-orbitals interact, they can interact with both lobes in the same phase or the opposite phase. This gives you two distinct types of an overlap.
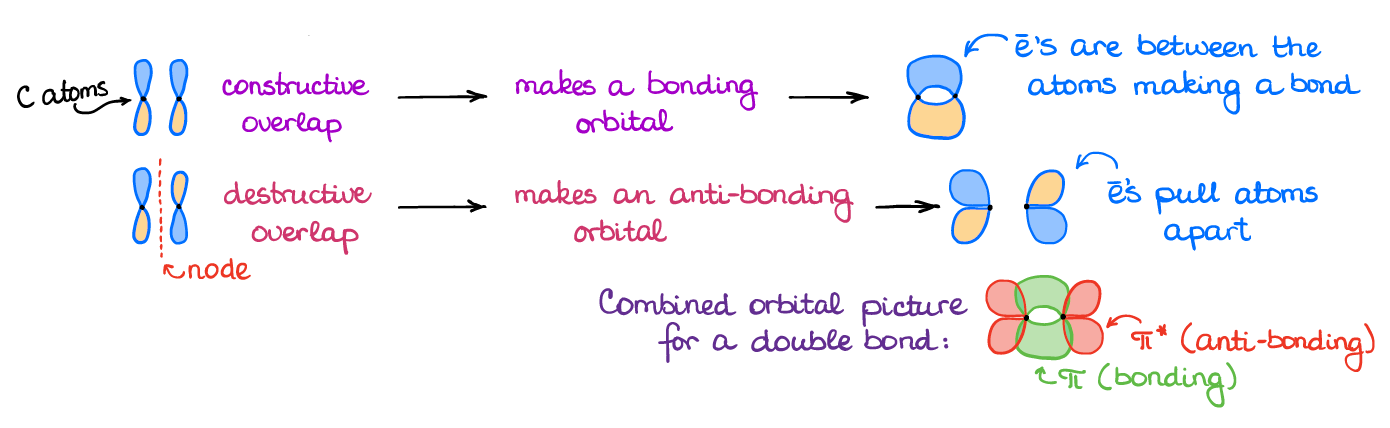
When the two p-orbitals are in the same phase, we have a “constructive” overlap. The constructive overlap creates a new area of the electron density in between the atoms creating a bond. When, however, we have the p-orbitals interacting while they are in the opposite phases, we have a “destructive” overlap, which creates the anti-bonding orbitals. If you were to put any electron density on the anti-bonding orbital, you would be counteracting the bonding effect of the bonding orbital. In other words, adding electrons to an anti-bonding orbital breaks bond. We won’t normally see electrons on those orbitals, unless we deliberately do something to the molecule to put electrons there. We’ll talk about the cases like that later. The take-home message here is that the two p-orbitals, when they interact, going to make two molecular π-orbitals: one bonding and the other—anti-bonding.
Number of Atomic Orbitals = Number of Molecular Orbitals
This simple mathematical principle is called the Local Combination of Atomic Orbitals (LCAO).
Energy of a Bonding and an Anti-Bonding Orbitals
The two π-orbitals that we create from the interaction of the p-orbitals are not equal in energy. The bonding orbital is lower in energy while the anti-bonding orbital is higher.
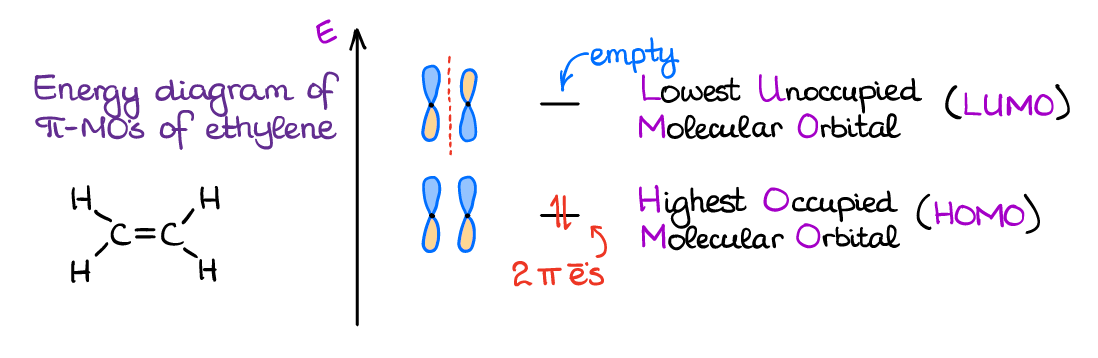
I also want to point out that the energy of the new bonding π-orbital is lower than the original p-orbital. Thus, it is energetically favorable to move electrons to this newly made orbital. The anti-bonding orbital is at a higher energy level; thus, we’ll keep it empty.
We’re also going to be focusing more attention on the very last orbital that has electrons on it. We’ll be calling such orbital the Highest Occupied Molecular Orbital (HOMO). The very next orbital, we call the Lowest Unoccupied Molecular Orbital (LUMO). Every interaction between the molecules in chemistry is going to be a HOMO-LUMO interaction. Thus, knowing the nature of the HOMO and LUMO orbitals will be important for many reactions of the conjugated systems.
How to Identify Bonding, Non-Bonding, and Anti-Bonding Molecular Orbitals
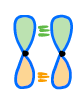
In a bonding molecular orbital, the interacting atomic orbitals (AO’s) are in the same phase. This type of interaction gives a constructive overlap and leads to the bond formation.
I also want to emphasize, that the bonding interaction can happen between multiple orbitals. This can give you bonding orbitals with 3 or more p-orbitals making the same bonding π-orbital.
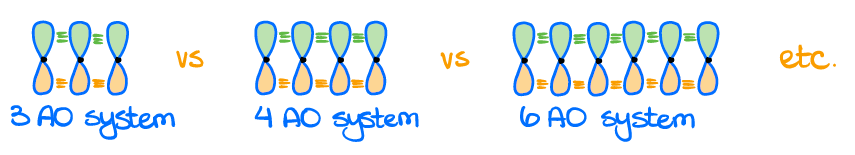
If you have the p-orbitals in the opposite phases, the overlap will be destructive. Such an overlap makes an anti-bonding orbital.
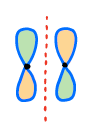
The area of space in between the atoms now has an orbital node. A node is the area of space where the probability of finding an electron is zero. Thus, no bonding can occur there.
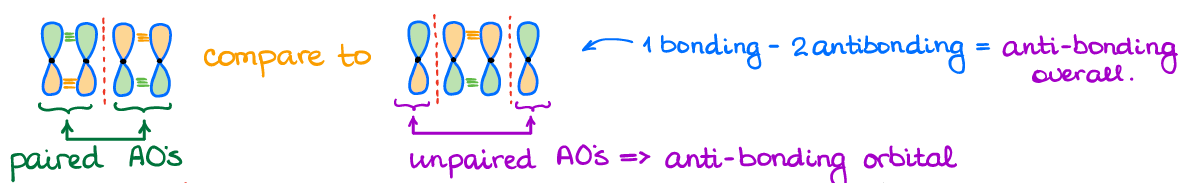
There’s something very important I want to mention here too. Just because an orbital has an anti-bonding interaction, does not make the entire orbital anti bonding. Bonding orbitals can have nodes. It is, however, all about how many of those nodes you have compared to the bonding interactions. If you have more bonding interactions, you get a bonding orbital. If you have more anti-bonding interactions, you get an anti-bonding orbital. Any anti-bonding orbital will also have unpaired p-orbitals. So, you can use that as a hint that you’re dealing with an anti-bonding orbital.
The non-bonding orbitals are, perhaps, the trickiest ones to identify. The non-bonding orbital must have a node (or nodes) where the p-orbital would normally be. This can only occur in the conjugated systems with an odd number of atoms in them. In this case, the p-orbitals are physically too far from each other to have any meaningful interaction.
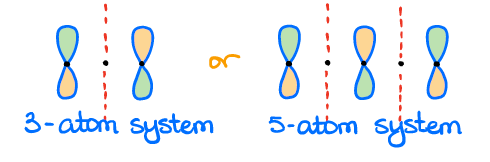
An important feature of the non-bonding orbitals is that they don’t have either bonding or anti-bonding interactions. So, these are rare. Another feature of the non-bonding orbitals is that they are right in the middle between the bonding and anti-bonding orbitals in terms of orbital energy. Thus, it’s neither favorable nor it is unfavorable to put electrons on those orbitals.
