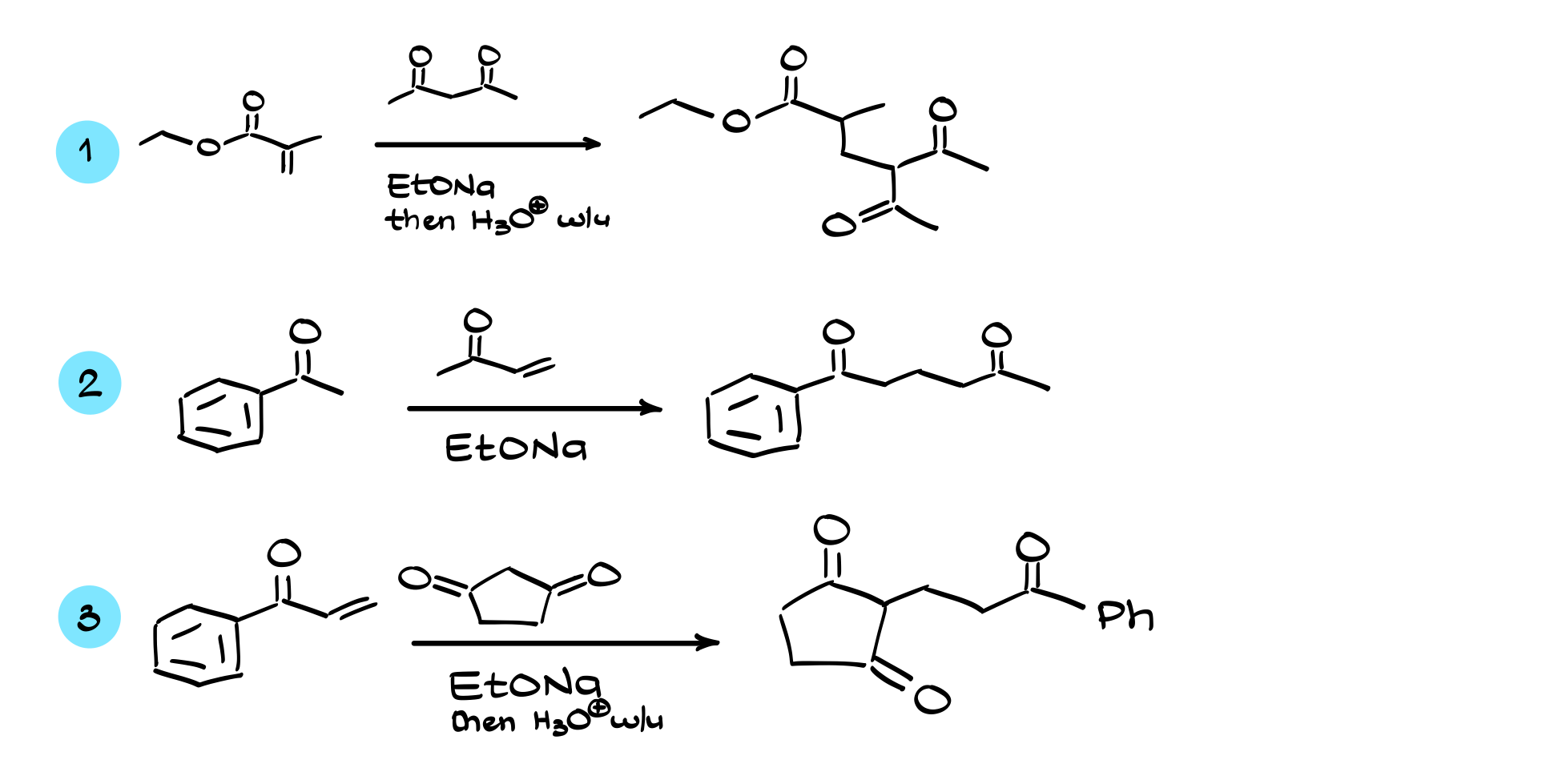
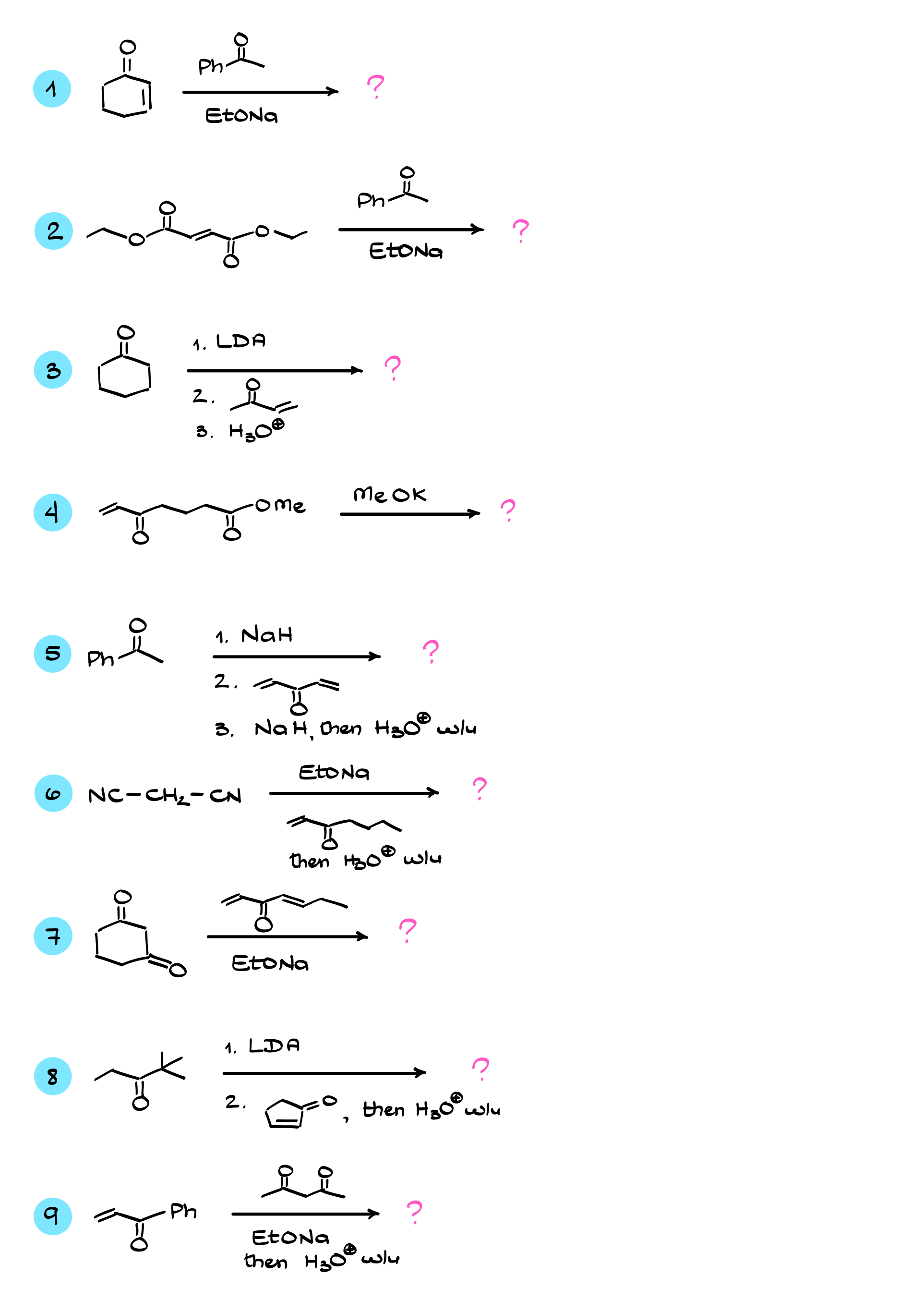
Michael Addition
In this tutorial, I want to talk about the Michael addition, which is a reaction between an ⍺,β-unsaturated compound and an enolate that we generate from some kind of carbonyl. As a result of this reaction, we typically end up with a 1,5-dicarbonyl product, which can either be a diketone or maybe an ester of some kind.

Conjugate vs Direct Addition to Conjugated Carbonyls
Before we dive into the details of the Michael addition mechanism, let’s first look at the two types of nucleophilic attack that alpha-beta unsaturated compounds can undergo.
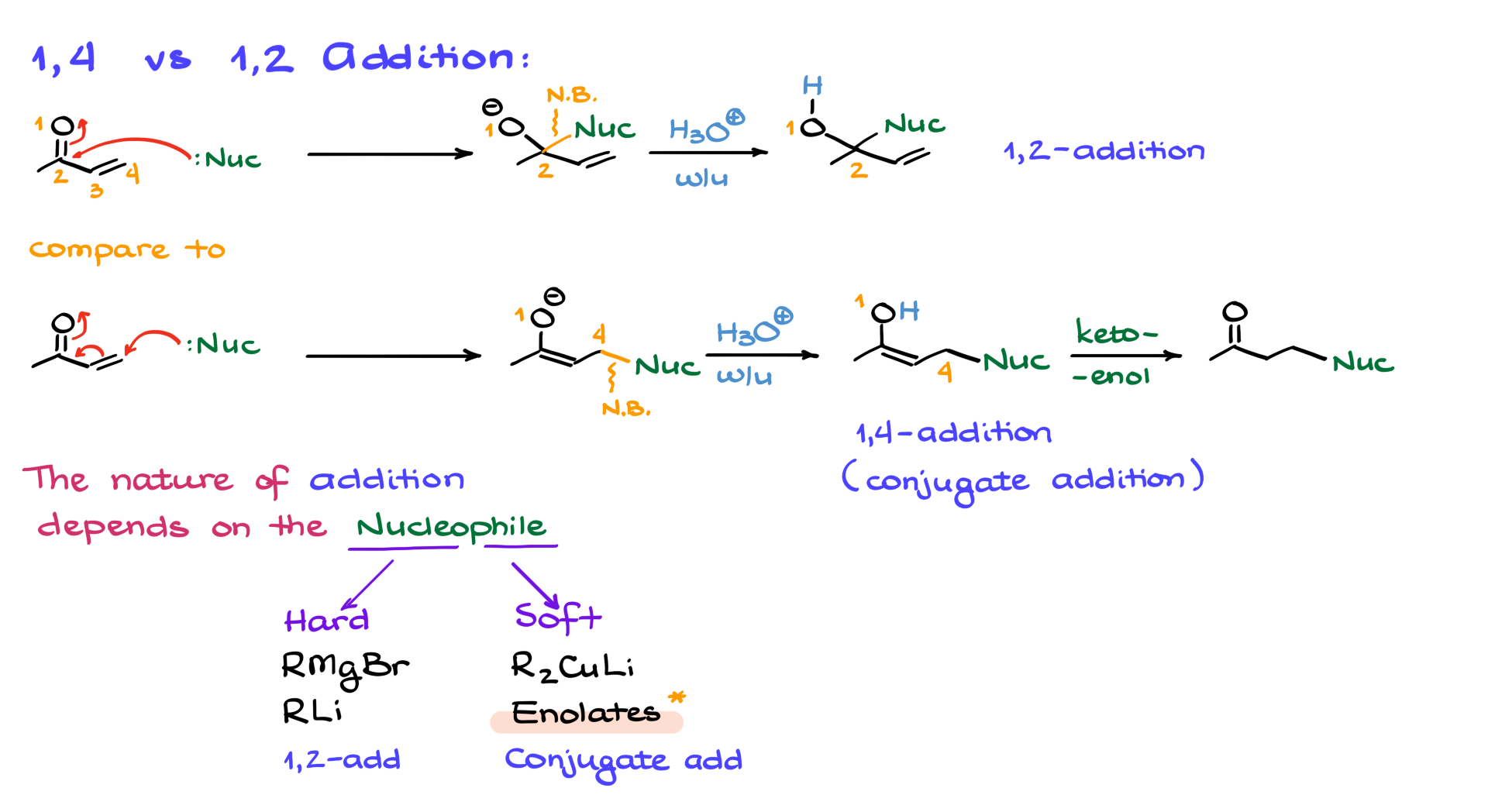
In one scenario, the nucleophile can directly attack the carbonyl, pushing electrons up onto the oxygen and forming a new carbon-nucleophile bond at the carbonyl carbon. If we number the atoms in our conjugated system starting from the oxygen as atoms 1, 2, 3, and 4, the nucleophile adds to atom number 2, which is the carbonyl carbon. After an acidic or aqueous workup, we’ll get a hydrogen at the oxygen (atom 1) and the nucleophile at carbon (atom 2). This kind of addition is commonly known as 1,2-addition.
However, there’s another possible pathway. The nucleophile can attack the beta position of the alpha-beta unsaturated compound, pushing electrons all the way to the oxygen and giving us an enolate intermediate. If we apply the same numbering system, the oxygen is still atom 1, but now the carbon attached to the nucleophile is atom number 4. After the acidic workup, the oxygen gets protonated, and we form an enol intermediate that quickly undergoes keto-enol tautomerism to yield the corresponding carbonyl. This second pathway is called 1,4-addition, although many textbooks and instructors now prefer the term “conjugate addition” because it’s less confusing and more accurately describes what’s happening in the reaction.
The type of addition—whether 1,2-addition or conjugate addition—depends heavily on the nature of the nucleophile. You’ll often hear nucleophiles described as either hard or soft. While this classification has its limitations, it’s still widely used, especially in the context of undergraduate chemistry courses. Hard nucleophiles tend to favor 1,2-addition and include species like organomagnesium compounds (Grignard reagents) and organolithium compounds. Soft nucleophiles, on the other hand, prefer conjugate addition. Examples of these include organocuprates like Gilman reagents and, most relevant for us, enolates.
Michael Donors and Michael Acceptors
Since we’re focusing on enolates, let’s talk about the Michael addition specifically. The conjugate addition of an enolate to an alpha-beta unsaturated compound is what we call a Michael addition. To better understand this, we need to introduce a couple of key terms: the Michael donor and the Michael acceptor. The Michael donor is the nucleophile, while the Michael acceptor is the electrophilic alpha-beta unsaturated compound. Almost any alpha-beta unsaturated carbonyl compound can serve as a Michael acceptor—whether it’s a conjugated ketone, ester, or even a diester. The notable exception is conjugated aldehydes, which tend to undergo 1,2-addition regardless of the nucleophile due to the high electrophilicity of the aldehyde carbonyl.
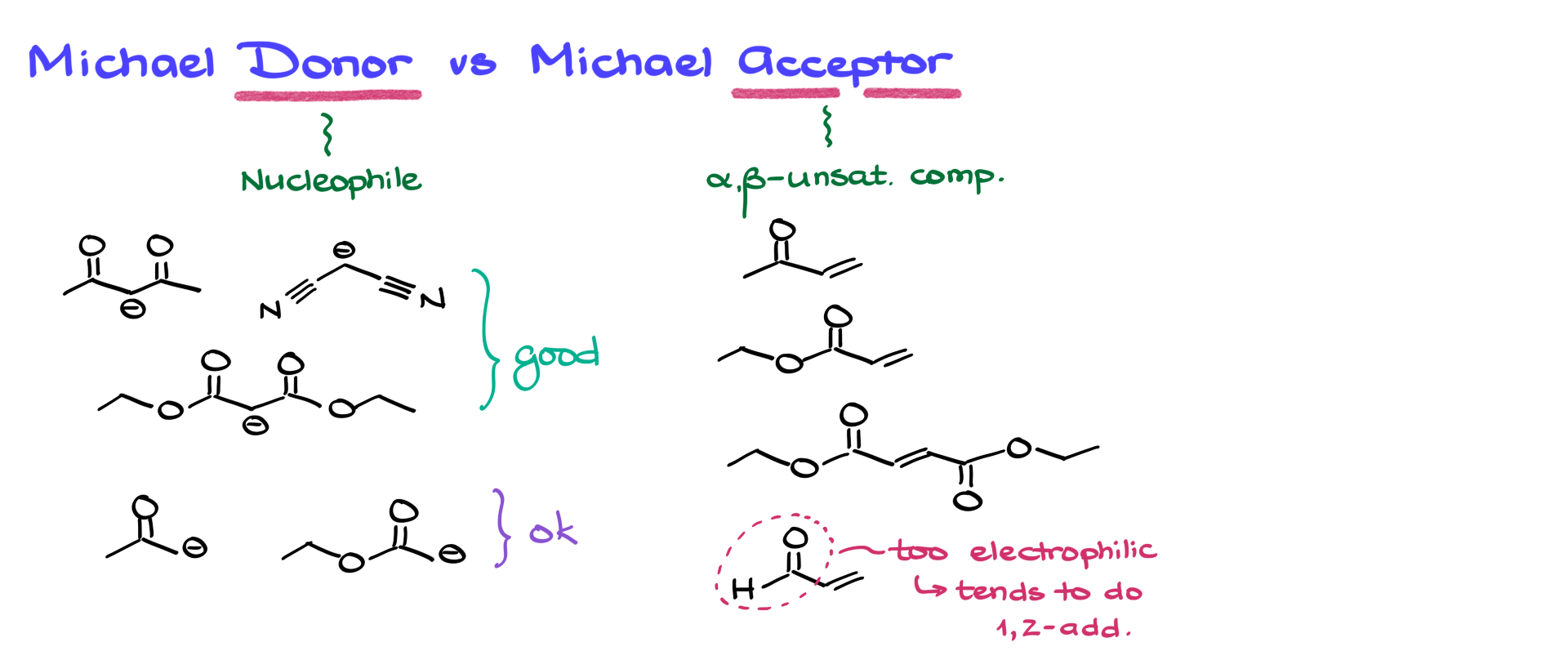
When it comes to Michael donors, not all enolates are equally effective. The more resonance-stabilized the enolate, the better it performs in the Michael addition. Enolates stabilized by at least two electron-withdrawing groups make for excellent Michael donors. By contrast, enolates with only one electron-withdrawing group can still work, but they’re more prone to side reactions, including 1,2-addition. Although it might seem counterintuitive that a more stable (and less reactive) nucleophile is better for this reaction, the explanation lies in the interaction of molecular orbitals—specifically, the HOMO and LUMO of the reacting species. This orbital interaction determines the reaction’s outcome, but understanding it fully goes beyond the scope of this tutorial. For now, just remember: the more resonance stabilization, the better.
Michael Addition Mechanism
Now let’s walk through the mechanism of a classic Michael addition. We’ll use a simple alpha-beta unsaturated compound reacting with malonic ester in the presence of sodium ethoxide as a base.
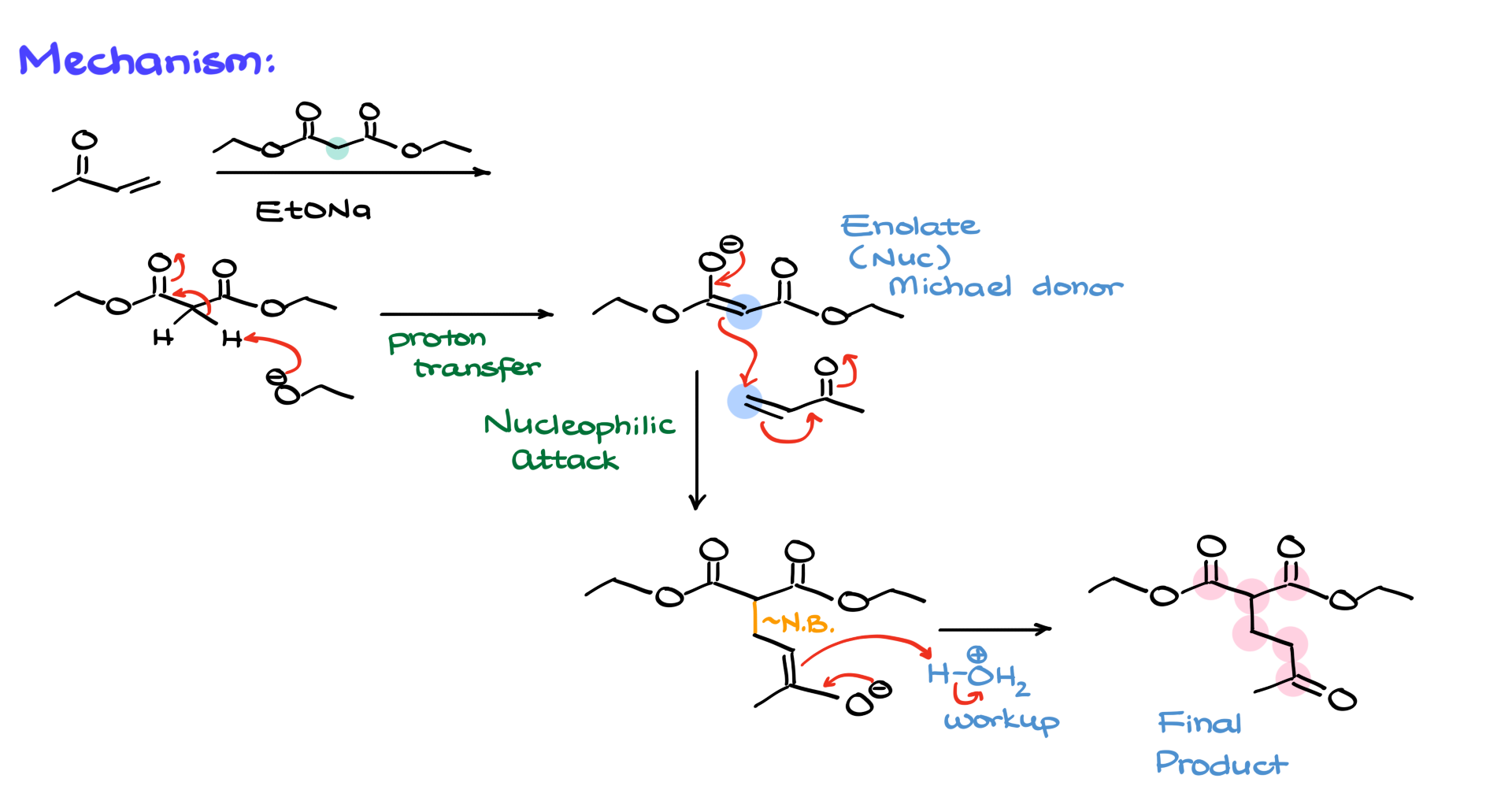
The first step is the formation of the enolate. Sodium ethoxide deprotonates the acidic alpha position between the carbonyl groups of malonic ester, giving us our enolate—a perfect Michael donor. Next, this nucleophilic enolate attacks the beta position of the ⍺,β-unsaturated compound, pushing electrons through the conjugated system and eventually onto the oxygen. This creates a new carbon-carbon bond between the ⍺-position of the enolate and the β-position of the electrophile, forming an enolate intermediate. Finally, an acidic workup protonates the enolate, yielding the 1,5-dicarbonyl product. If you count the atoms from one carbonyl to the other, you’ll find exactly five carbons, which is a hallmark of the Michael addition.
Michael Addition Examples
Let’s go through some examples to reinforce the concept.
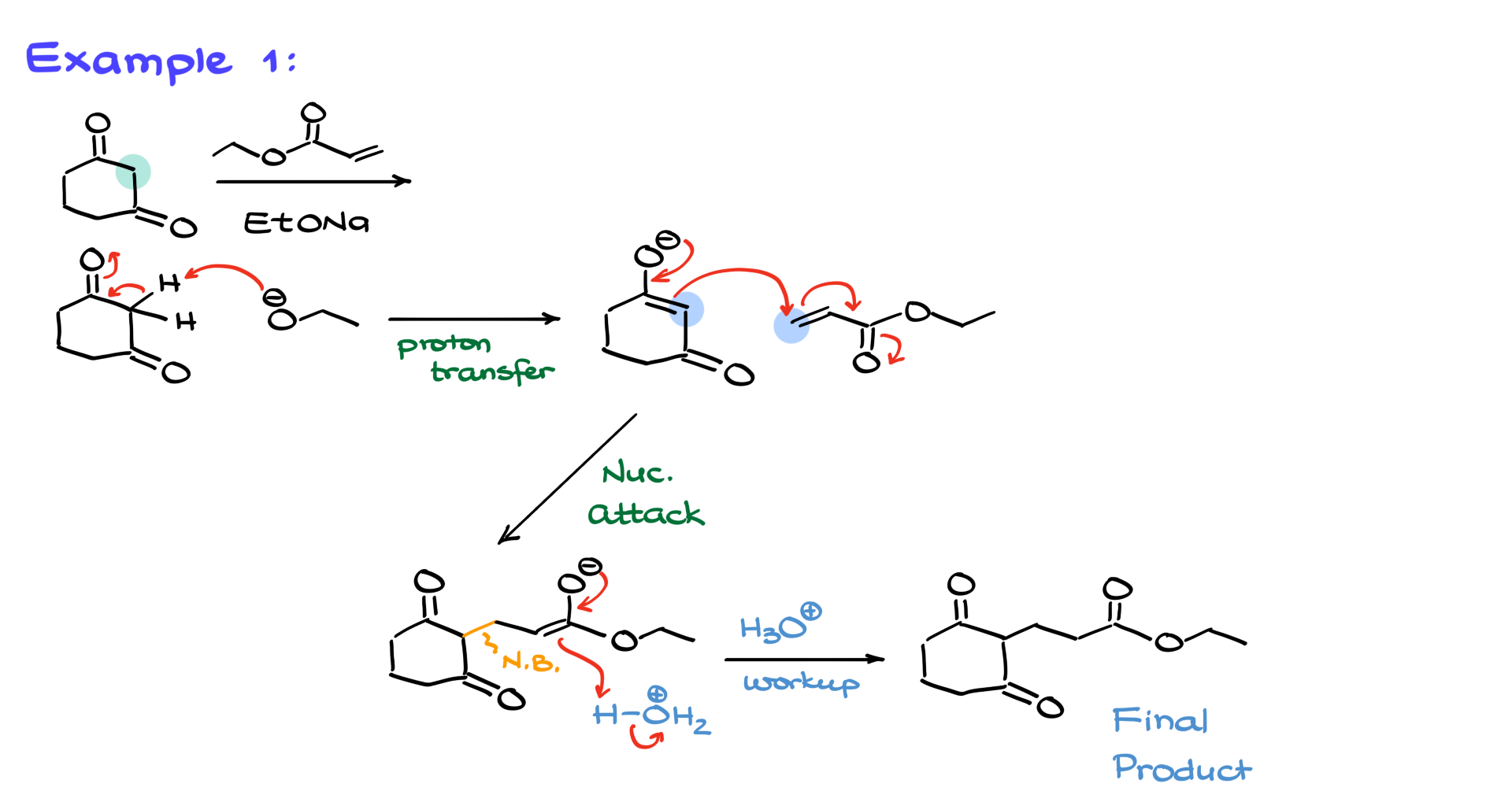
In one case, we start with a 1,3-dicarbonyl and react it with an alpha-beta unsaturated ester in the presence of a base. As usual, we begin by identifying the most acidic proton—it’s the one between the two carbonyl groups. The base deprotonates this position, forming our enolate nucleophile. Then, the enolate attacks the beta position of the unsaturated ester, forming the 1,5-dicarbonyl product after the acidic workup.
Here’s one more example:
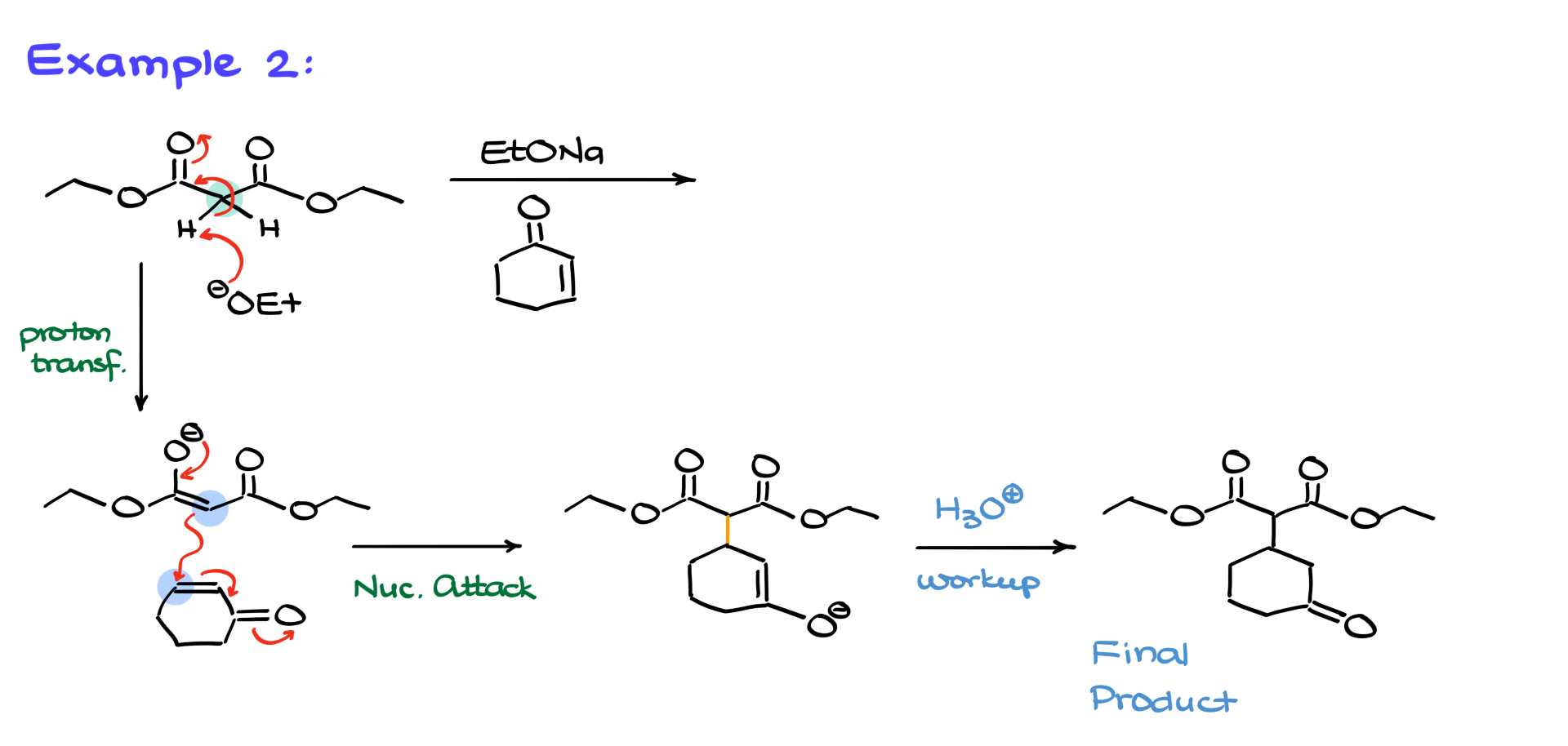
Intramolecular Michael Addition Examples
In another example, I have an intramolecular Michael addition.
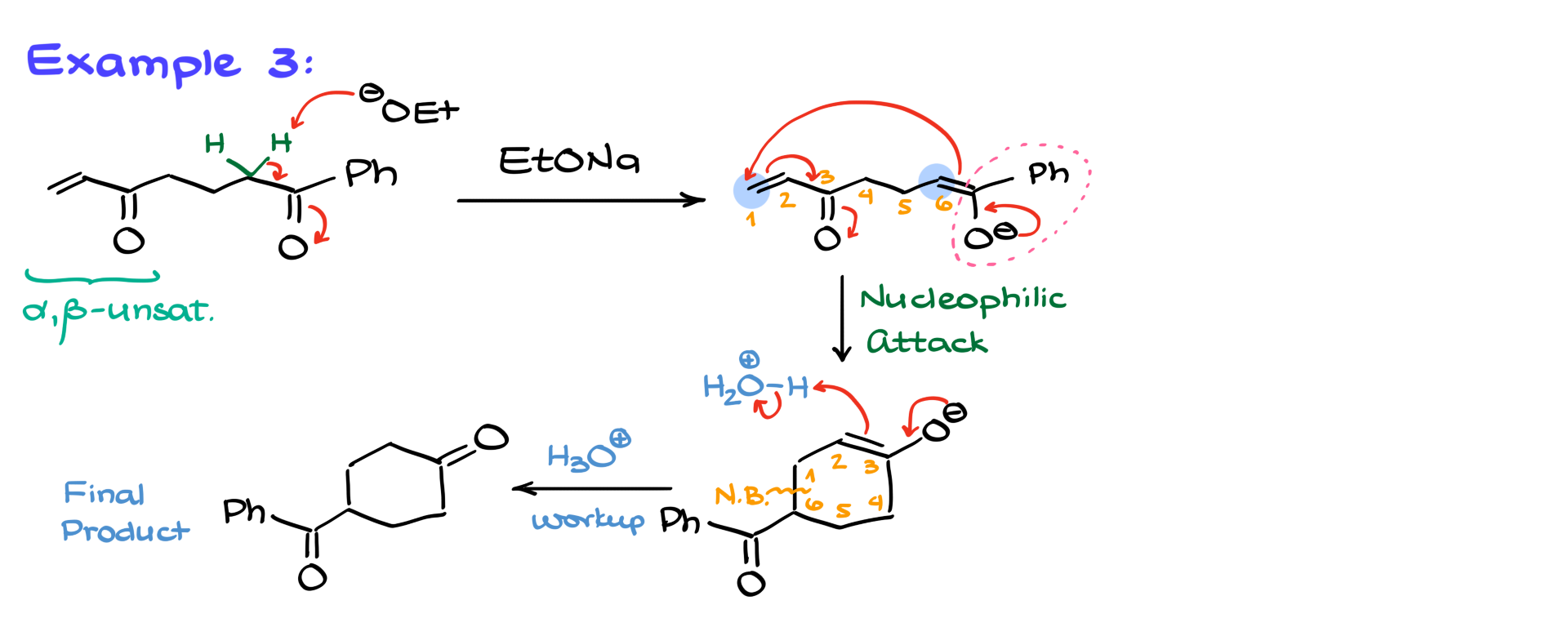
Here, the Michael donor and acceptor are part of the same molecule. The base again deprotonates the most acidic proton, forming an enolate. This enolate then attacks the intramolecular alpha-beta unsaturated site, forming a new carbon-carbon bond and, ultimately, a six-membered ring after the final acidic workup. Intramolecular reactions like this are quite common and can sneak up on you during exams, so always watch out for the possibility of intramolecular cyclization when both a donor and acceptor are present in the same molecule.
Finally, let’s look at a more complex scenario: a molecule with two alpha-beta unsaturated groups—essentially a double Michael acceptor.
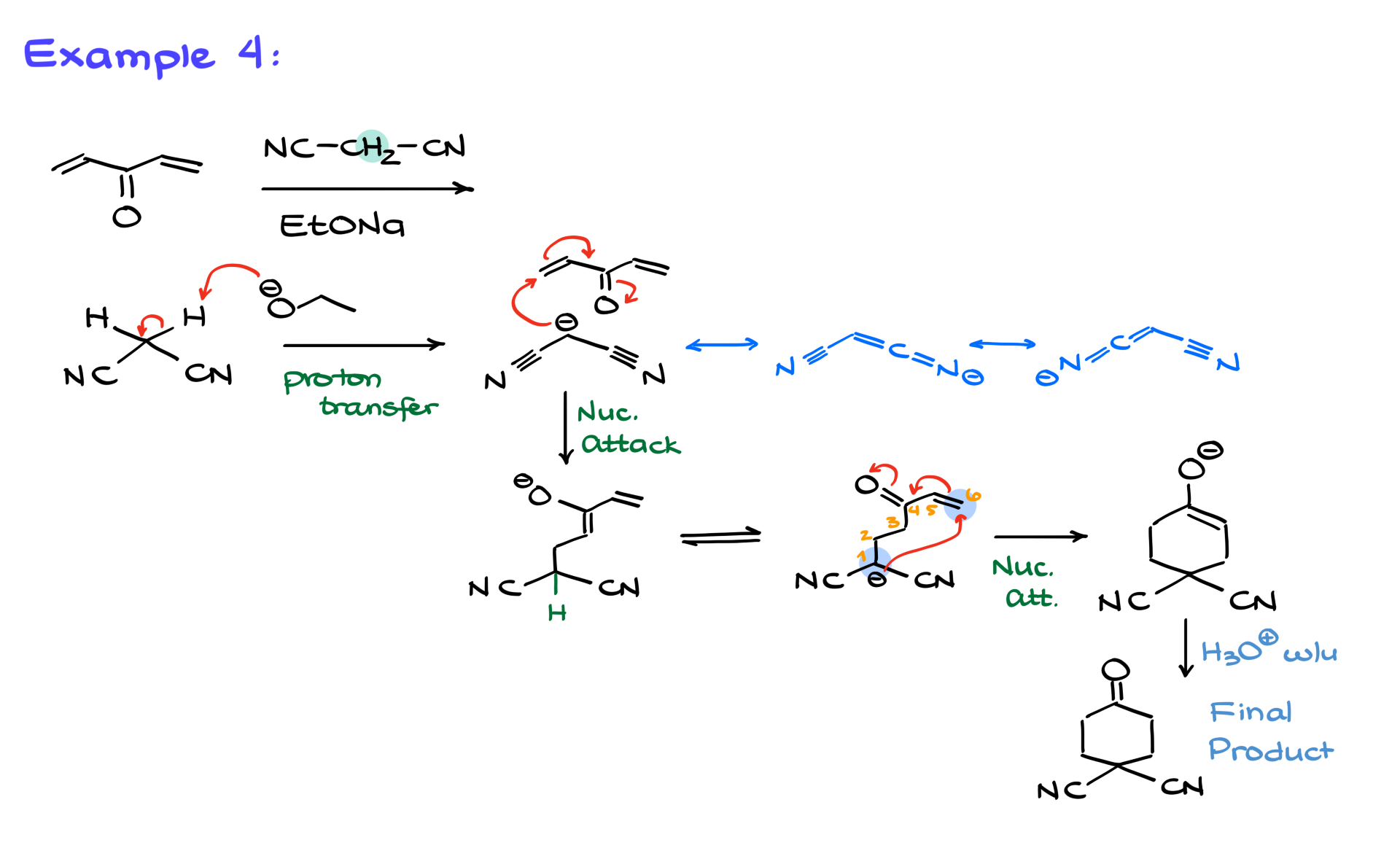
The base first deprotonates the carbon between two nitrile groups, creating a resonance-stabilized anionic species. This enolate then attacks one of the Michael acceptors, forming a new carbon-carbon bond. But because there’s a second Michael acceptor present, the reaction doesn’t stop there. The intermediate can undergo a second Michael addition, ultimately forming a bicyclic structure after the acidic workup.
So what do you think about the Michael addition? It’s a powerful and versatile tool in synthetic organic chemistry, and you’re almost guaranteed to see it on your final exam. Make sure you understand the mechanism and can recognize when a Michael addition might occur, especially if there’s an intramolecular component involved.
Michael Addition Practice Questions
Would you like to see the answers and check your work? Become a member today or login if you’re already a member and unlock all members-only content!