Markovnikov’s Rule
Imagine yourself walking through the dining hall. What are you going to choose? A pizza or a salad? When you’re choosing between pizza and salad, you instinctively know where you’ll end up. In the world of molecules, Markovnikov’s Rule helps us predict similar choices.
So, what exactly is Markovnikov’s rule and why is it one of the most misunderstood rule in organic chemistry? Let’s dig in!
The Origins of the Markovnikov’s Rule

In the 1870’s Russian chemist Vladimir Markovnikov published his observations of the outcomes of the hydrohalogenation of alkenes. He observed that when alkenes react with hydrogen halides like HCl, HBr, or HI, the halide typically ends up on the more substituted atom of the double bond, while the hydrogen—on the least substituted. And to make sure we’re crystal clear on the terminology, the more substituted atom is the one that is connected to more other atoms that are not hydrogens. So, if the a carbon atom is connected to three other carbons, it’s more substituted than the one that’s only connected to two other carbon atoms.
In the times of Markovnikov, chemists didn’t know about the carbocations and had a very vague idea of the mechanisms of organic reactions in general. It would take over 50 years for chemists to develop the first idea of the mechanisms and what’s happening in the reactions. So, at the time of Markovnikov chemists could only describe what they saw experimentally. But they really couldn’t explain their observations. Now, of course, we know better.
So people started calling products with the halide on the more substituted atom the Markovnikov’s product. While the molecules with the halide on the less substituted atom were appropriately called the anti-Markovnikov’s product. This notion slowly but steadily spread to other reactions and people started calling any molecule with the group of interest on the more substituted atom as the Markovnikov’s product. An now we often come across the reactions that make Markovnikov’s alcohols, or Markovnikov’s halohydrins, or Markovnikov’s vicinal dihalides, etc. Which naturally makes it very confusing what exactly that entails in every case.
Now, I’m going to tell you something very important here. Markovnikov’s rule, just like the anti-Markovnikov’s rule do not exist. They are a fiction. Likewise, it’s really silly to talk about the Markovnikov’s or anti-Markovnikov’s products. Both rules follow the same fundamental mechanistic principle of organic chemistry: in any reaction, we’ll always form the most stable reactive intermediates. And for as long as you follow this fundamental principle when working through your reactions, you’ll always come up with the correct product and won’t ever need to memorize whether the reaction follows the Markovnikov’s rule or otherwise. This of course, means that you need to have a firm understanding of the mechanisms of the reactions you’re working through.
Mechanistic Principles Behind the Markovnikov’s Rule
Today, we know that the Markovnikov’s rule boils down to one simple principle: stability. So, if I took a molecule, 2-methylbut-2-ene, and reacted it with hydrogen halide, like HBr, the firs step can produce two different carbocations: the tertiary (3°) and the secondary (2°) carbocation. And as we know, the tertiary carbocation is more stable than the secondary one. Thus, we’ll keep that one and discard the secondary one. Does that mean that we do not make any quantity of the secondary carbocation? No, it does not. The secondary carbocation will show up. But it will be present in a very small quantity. The greater the stability difference between our possible intermediates, the greater the ratio we’ll see for them. For something like a secondary vs tertiary carbocation, we’ll have hundreds of thousands if not millions of the tertiary carbocations forming for each secondary one in a given system.
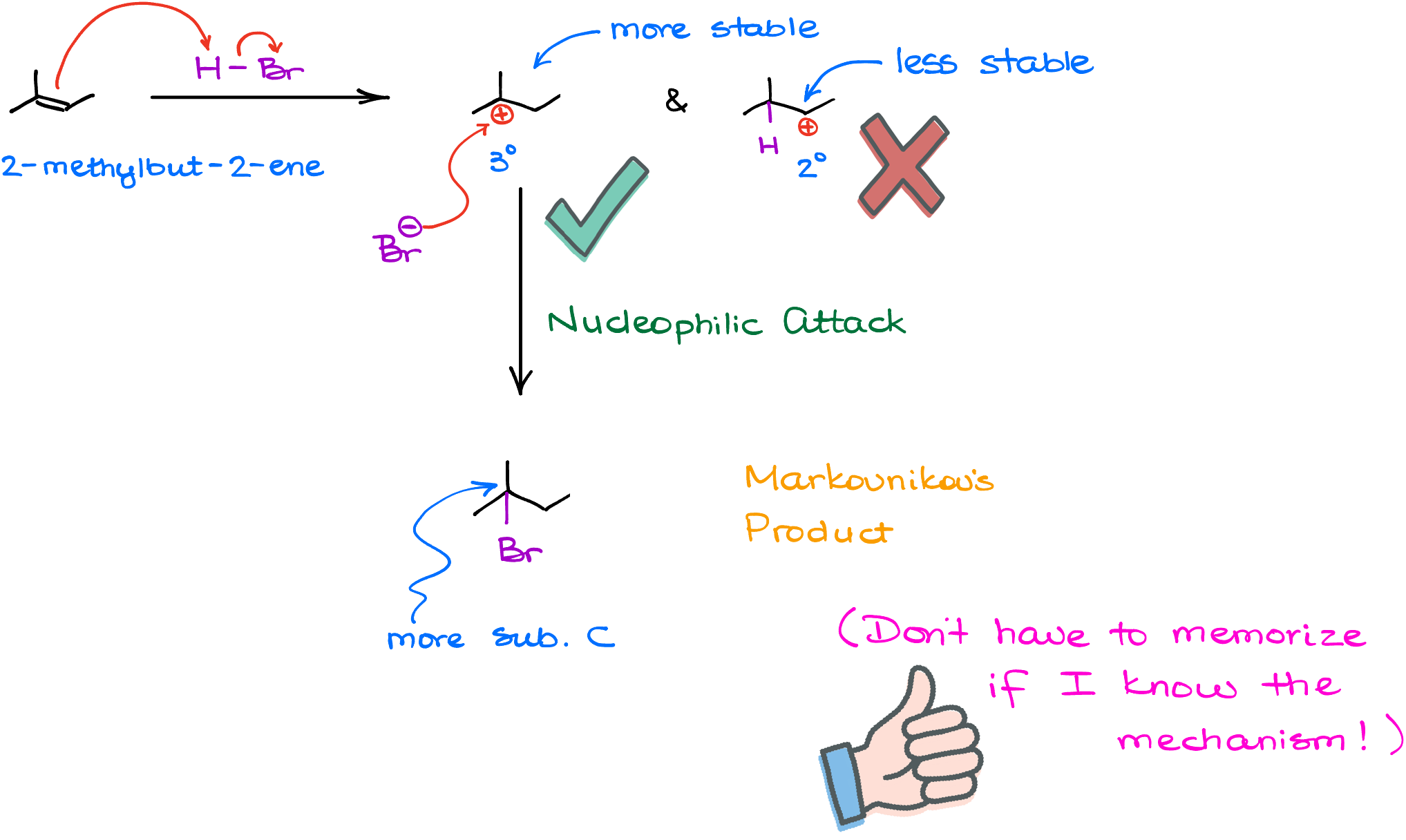
So, because of the greater stability of the tertiary carbocation in this example, we’ll ignore the minuscule amount of the secondary carbocation intermediate. And in the next step, when the halide anion attacks our carbocation, we’ll end up with the final product where I have the bromine on the more substituted atom. And so I don’t have to remember that this reaction follows the Markovnikov’s rule. I just have to remember that all reactions make the most stable intermediate.
Anti-Markovnikov’s Addition
So you’re thinking, “What about anti-Markovnikov’s hydrohalogenation?” Don’t worry, this reactions is not a rule-breaker either. It just plays a different game—radical mechanisms! And despite the fact that we have a different mechanism, we still follow the same fundamental principle: we make the most stable intermediate.
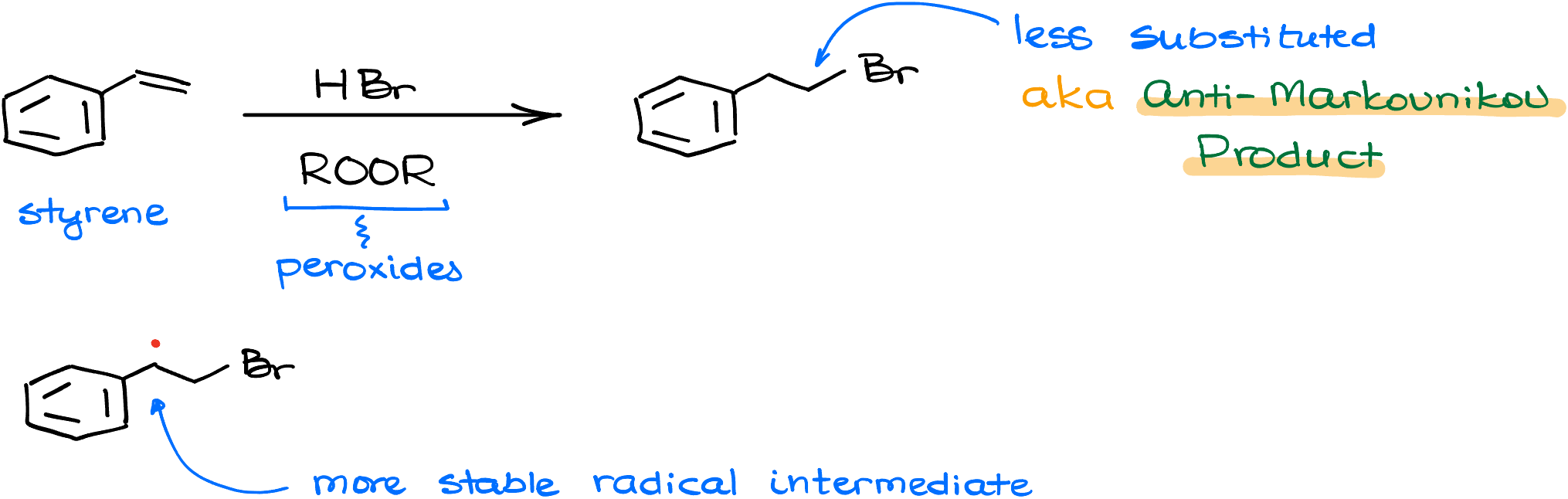
Let’s say I’ll react styrene with hydrogen bromide in the presence of organic peroxides. This reaction indeed produces the major product with the bromine on the less substituted atom. But if we look at the intermediate of this reaction—a radical—we’ll see that this radical is on the more substituted carbon. Carbocations and radical follow the same stability pattern. The more substituted radical is more stable. So, we still keep true to our fundamental principle of making the most stable intermediate.
How to Quickly Predict Products of Reactions Using the Markovnikov’s Rule
Of course, working through the mechanism every single time is tedious and takes a lot of time. If you’re pressed for time, here’s a quick tip: the electrophile typically goes to the less busy carbon atom, while the nucleophile chooses the more substituted one. But remember, knowing the “why” is better than just memorizing! I always advice to practice your mechanisms regardless, so you can quickly run through the mechanism of any reaction in broad strokes in your head. This way you’ll always get the correct product. And if your functional group is on the more substituted atom, call it “Markovnikov’s product.” Likewise, if your functional group is on the less substituted atom, that’s the anti-Markovnikov’s product. As simple as that.
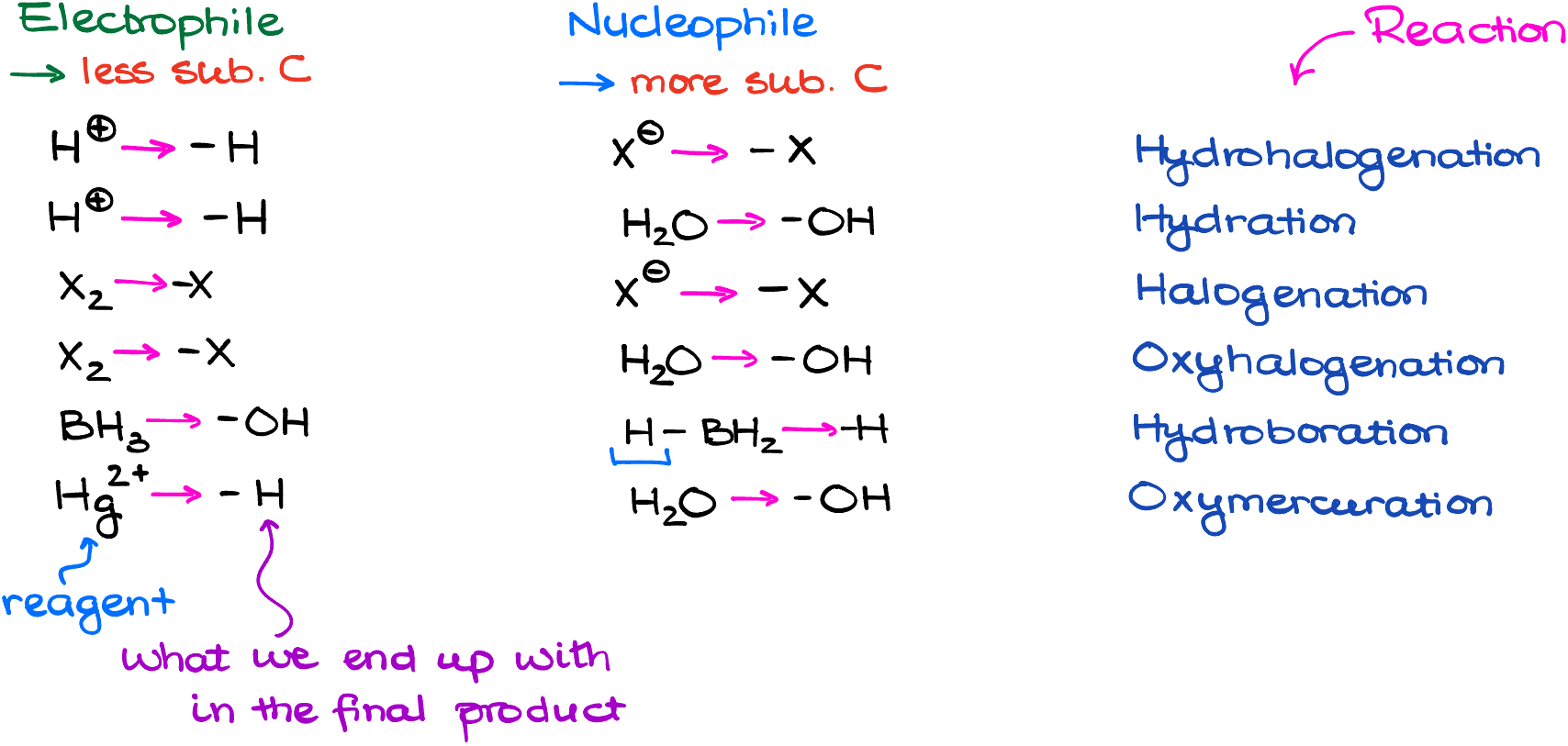
That means that you still need to know your mechanisms and know what is the nucleophile and what is the electrophile in each reaction. But if you try to blindly memorize which reaction is Markovnikov and which is anti-Markovnikov, you will eventually get overwhelmed and confuse those when it matters the most. You can write down the table below as a quick reference guide for the most common reactions. But remember, memorization will never trample understanding, so rather than trying to memorize it, always emphasize they “why” factor and consciously to think mechanistically about your reactions.
So there you have it. Markovnikov’s Rule isn’t as mysterious as it seems. With a little practice, you’ll be predicting those reaction outcomes like a pro!

OMG! This is by far the best explanation! I’m sick of my professor constantly telling us just memorize this rule and memorize that rule! I’m here to understand not to memorize! Thank you so much!
Thank you, Jackie! That’s what I’m all about as well–understand, not memorize!