Keto-Enol Tautomerism
Keto-enol tautomerism is one of those interchapter topics that surfaces throughout organic chemistry course several times. The first time you’ve seen the keto-enol tautomerism was in the alkynes chapter when you were learning about the hydration of alkynes or hydroboration-oxidation of alkynes. But where we truly dive deep into the intricacies of the keto-enol equilibrium is when we start talking about the chemistry of enols and enolates.
What is Keto-Enol Tautomerism?
Keto-enol tautomerism is an equilibrium between two constitutional isomers that differ from each other by the location of a proton in the molecules. True to its name, keto-enol tautomerism is the equilibrium between a ketone (or an aldehyde) and a corresponding enol.

There are many different factors that influence this equilibrium. Also, the transformation from the ketone to enol and back can be catalyzed by both basic and acidic conditions. So, let’s look at each of these conditions and the list of factors a little closer.
Keto-Enol Tautomerism in Basic Conditions
I have a tutorial on the enolization of carbonyls in basic conditions. In that tutorial I described how we can use different bases to enolize various carbonyls. If we have a source of protons, however, instead of an enolate we can get an enol.
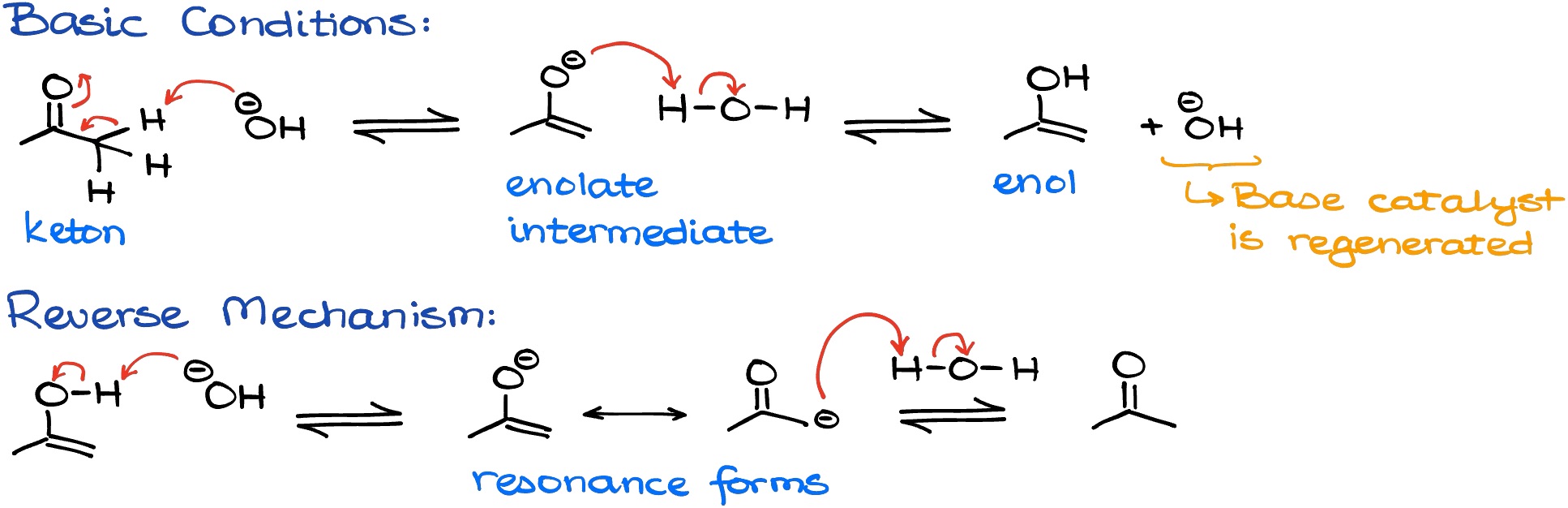
However, due to the acidity of enols, we typically don’t see the actual enol in solutions with any non-negligible concentration of the base. If, however, we use the base in a very small catalytic quantity, then we can get enolate in measurable quantity.
Keto-Enol Tautomerism in Acidic Conditions
Keto-enol tautomerism can be catalyzed by the acidic conditions as well.
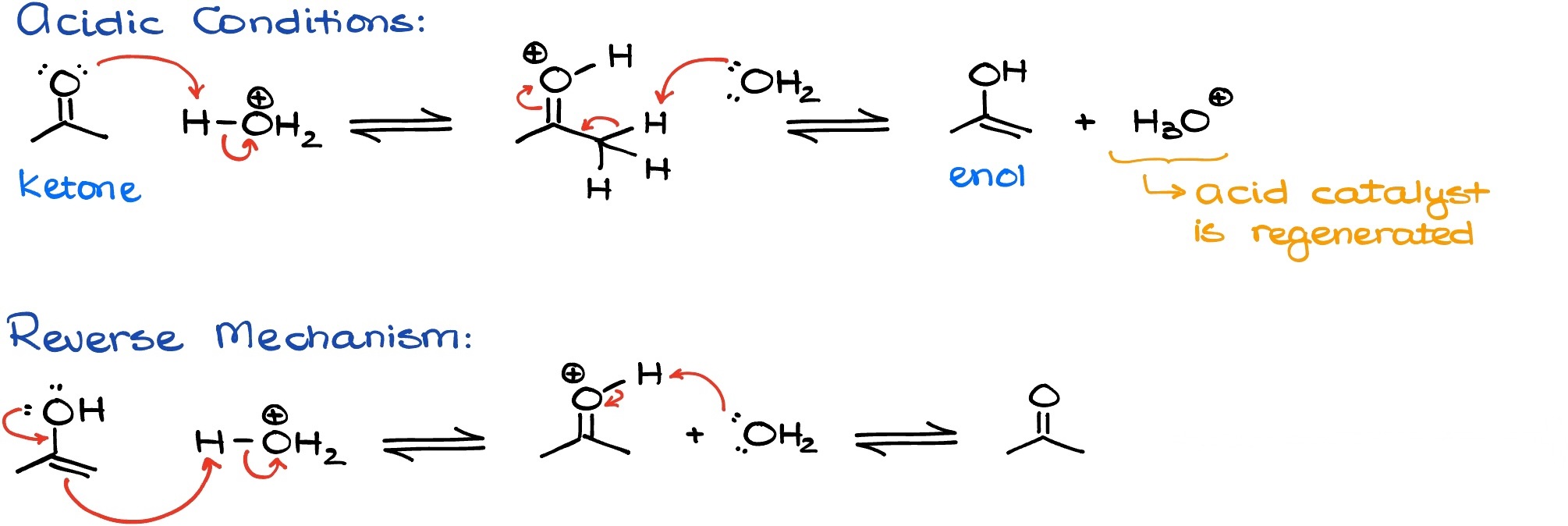
Typically, acid is always going to be used in a small catalytical quantity. The difference here is that we are never going to see the enolate in this equilibrium.
Factors Affecting Keto-Enol Tautomerism
Since keto-enol tautomerism is an equilibrium process, we will favor the side with more stable species. Thermodynamically, the keto form (either a ketone or an aldehyde) is typically more stable.

However, there are factors that may make the enol form more stable. Those are:
- Extended conjugation, and
- Hydrogen bonding.
Let’s, for instance, look at acetylacetone (pentane-1,3-dione). This compound exists in an equilibrium between the keto form and the enol form.

Interestingly, in this case the enol form predominates in the solution, which can be confirmed by HNMR spectroscopy and other methods. Why is this molecule more stable in the enol form? The two reasons I’ve mentioned earlier: conjugation and hydrogen bonding.

As the matter of fact, most β-dicarbonyls exist in the enol form, especially the diketones. However, traditionally, we still show them all in the keto form regardless of how they look like in reality. So, unless your instructor specifically asks you to keep some of those in the enol form, always draw the dicarbonyl version of the molecule. This is also the way your textbook is going to represent them in the vast majority of cases.
Thermodynamic Enols are Always Favored
We have seen before that enolization of carbonyls can lead to the formation of multiple different enolates. In the case of the keto-enol tautomerism, the situation is similar.
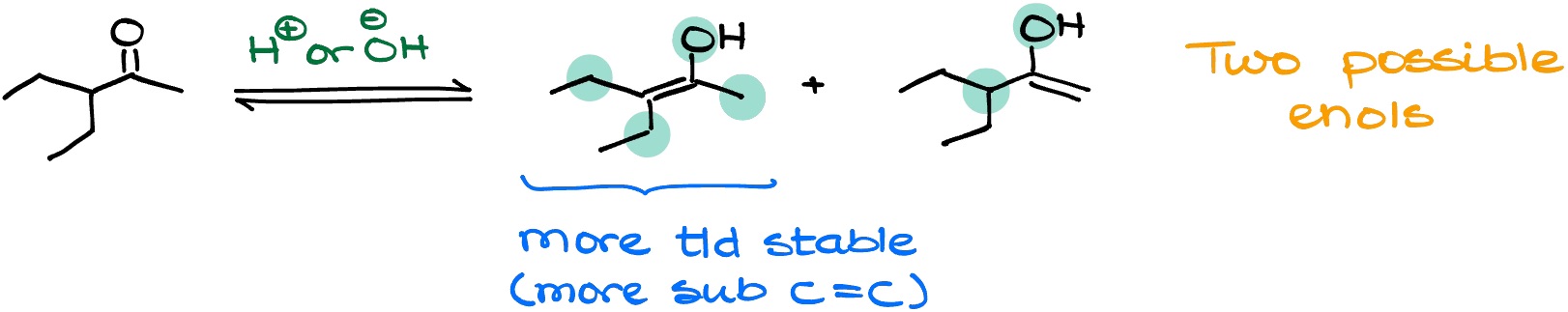
When we have a ketone that can potentially give you two (or more) different enols, you should always choose the more thermodynamically stable one as your most predominant enol. And like before, the more thermodynamically stable enol is typically the one with the more substituted double bond. However, keep in mind that this is an equilibrium, and all possible enol forms will be present. So, we might occasionally use the less prevalent enol for some reaction in the future if the overall equilibrium necessitates the involvement of such an intermediate.
