Jones Oxidation
Jones oxidation is, probably, one of the most iconic oxidation reactions of alcohols. And depending on the structure of the starting alcohol, Jones oxidation can yield either a carboxylic acid or a ketone.
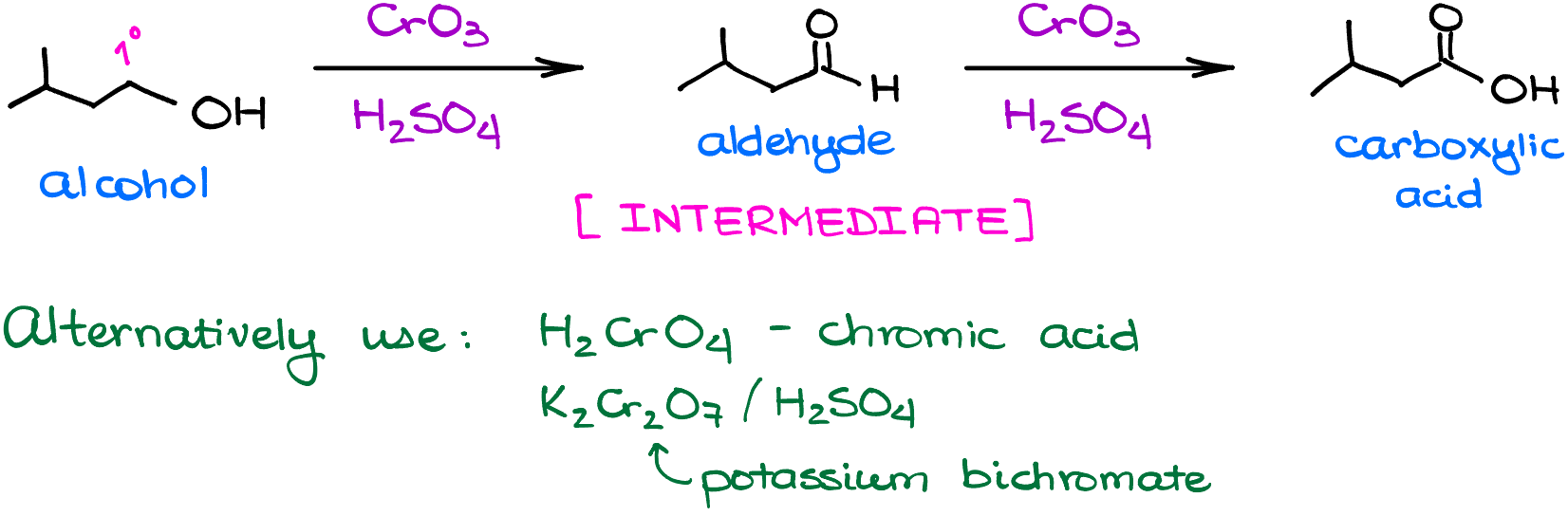
The traditional Jones oxidation is a reaction of an alcohol with a mixture of chromium oxide (CrO3) and dilute sulfuric acid (H2SO4) in a water-acetone solvent mixture. And while this is a classic variation, there are some other versions that you may encounter in your course. For instance, some people like to perform this reaction with chromic acid (H2CrO4) or potassium bichromate (K2Cr2O7) in sulfuric acid. You can use whichever reagent you like most because they all perform the same function here. So, for the rest of this tutorial I’ll be just using the “classic” version with the chromium oxide and sulfuric acid. But remember, that these reagents are interchangeable.
Overview of the Oxidation Reactions
The nature of the alcohol will influence the outcome of the oxidation reaction. Primary (1°) alcohols can give you either aldehydes, or if the reaction keeps going—carboxylic acids. Jones oxidation is one of those reactions that keeps going. So, while the aldehydes will be products in this reaction, they are going to be intermediates rather than final products.
When it comes to the secondary (2°) alcohols, they always make ketones regardless of which oxidation method you’re going to choose.
One common mistake I see many students make is they add a carboxylic acid to a molecule whenever they see the Jones oxidation conditions. Keep in mind, that in this reaction, we’re oxidizing existing carbons, so don’t add extra carbons to your molecule.
Mechanism of the Jones Oxidation
Since we now know what to expect from our reaction, let’s look at the actual mechanism of the reaction of the Jones oxidation. As the reaction is performed in the aqueous solution of chromium oxide in sulfuric acid, we’ll start by reacting the alcohol with the protonated chromium oxide. Then, after the proton transfer, in which we’re going to use water as a base, we’re going to end up with an intermediate where we have chromium sitting on the oxygen atom instead of the hydrogen that used to be there.
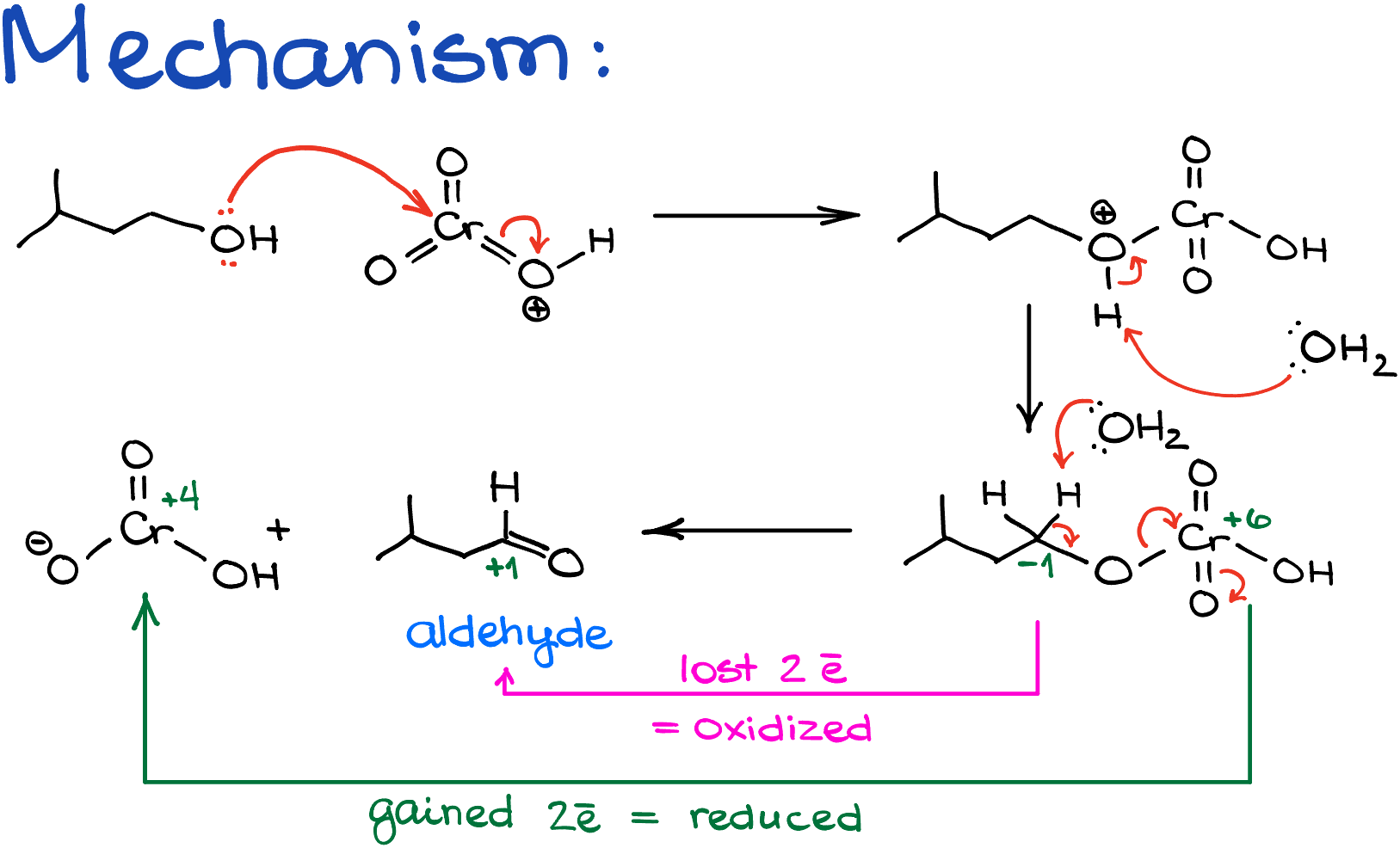
Next, water comes into play again, deprotonating the alpha hydrogen, giving us the aldehyde. This step is the red-ox step itself. In this case, the oxidation state of carbon goes from -1 to +1, and the oxidation state of chromium goes from +6 to +4. So, carbon formally loses the electrons, while chromium gains those electrons.

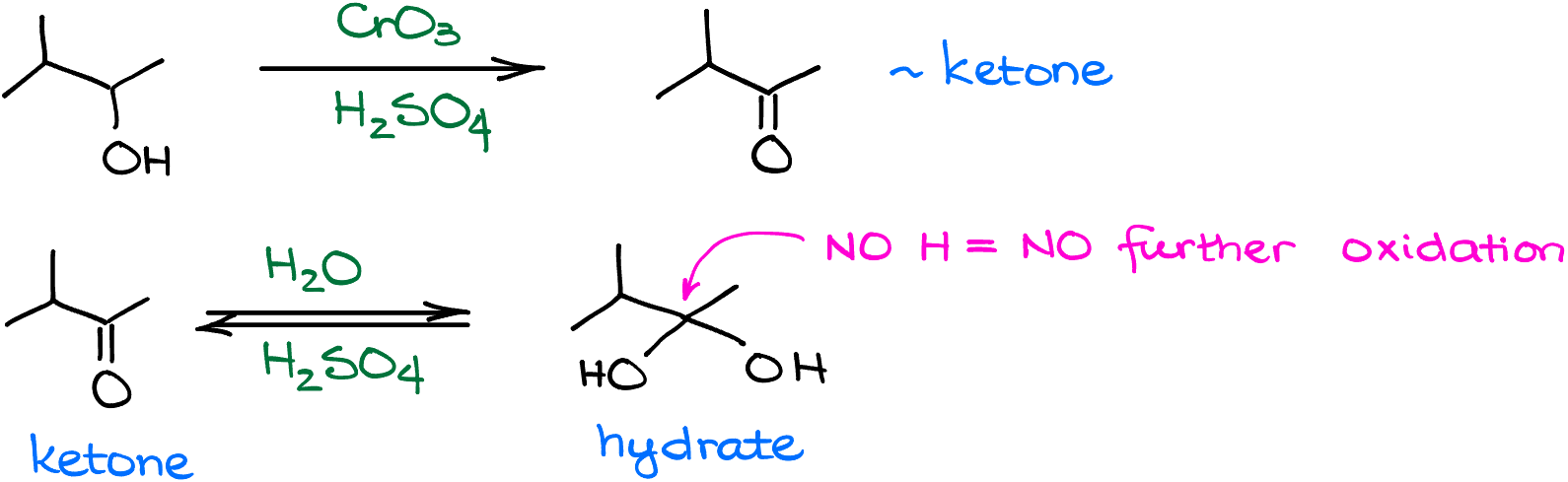
And since we’re working in protic aqueous conditions, the aldehyde intermediate will get protonated and will quickly react with water. After the intermediate loses the proton to the environment, we’re going to have a hydrate—a molecule with two -OH groups on the carbon. This is an equilibrium process, and the reaction can go back to the aldehyde form. But here’s the catch, as soon as we form a hydrate, it can now undergo another round of oxidation since the oxidizing agent is still floating around.
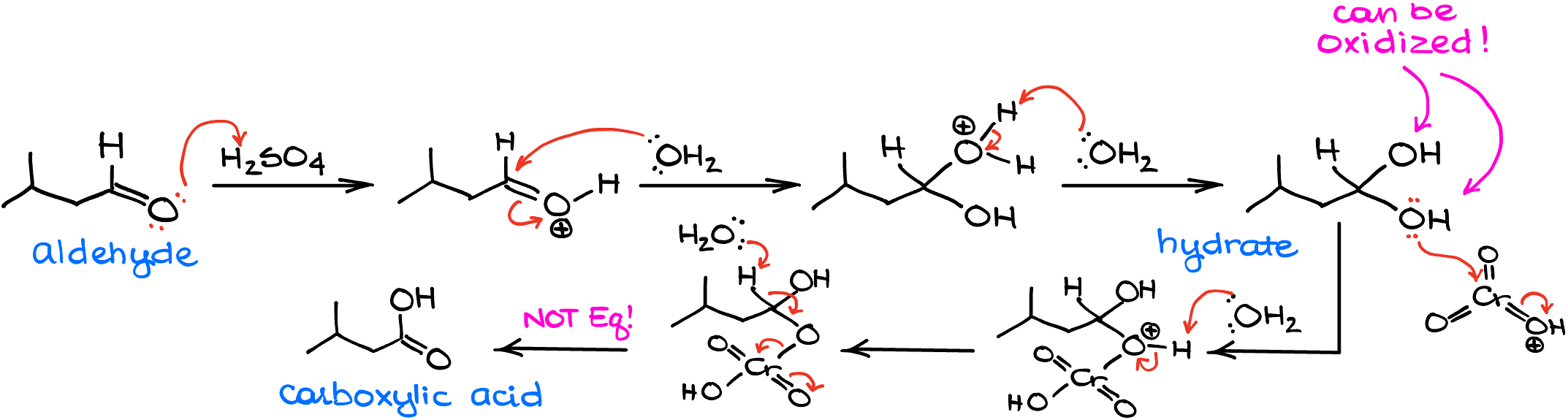
So, once chromium oxide “catches” our hydrate, the next round of oxidation begins. The mechanism in a nutshell is the same as in the first round. Here, we’re going to form an intermediate in which one of the -OH groups will have the chromium bit hanging off it. And like in the first round, we’re going to use water to facilitate the last step of the oxidation process giving us the carboxylic acid.
The oxidation step, however, is not an equilibrium process. So, once we’re at the stage of the carboxylic acid, there’s no going back! And because of that, Jones oxidation always takes your starting primary alcohol all the way to the carboxylic acid and never yields an aldehyde as the final product.
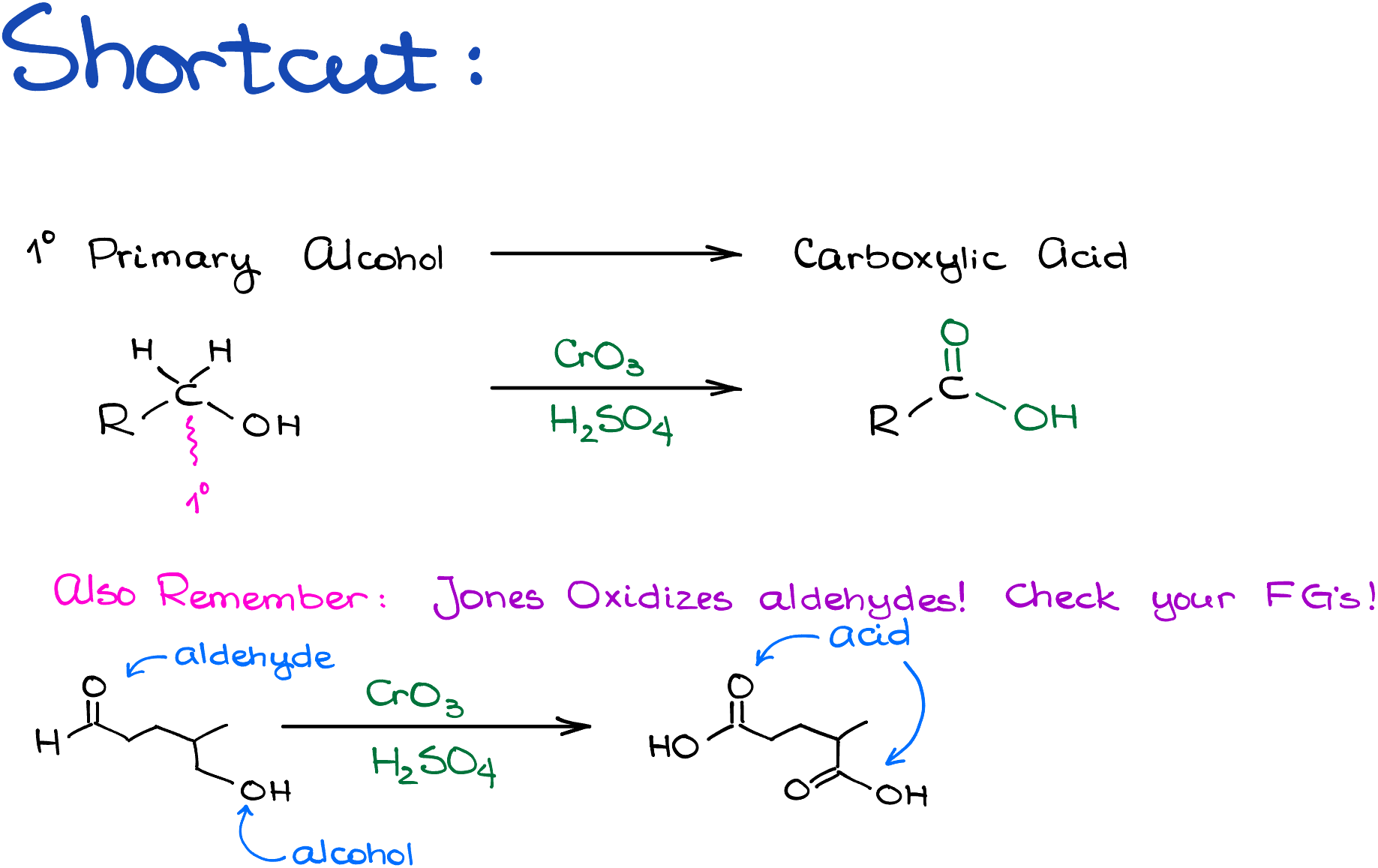
Moreover, if you have an aldehyde in your starting material, we’re going to oxidize it in the process to the carboxylic acid as well in addition to the oxidation of your alcohol. So, check your functional groups carefully. Throwing an aldehyde here and there onto your molecule is a common trick some instructors like to employ, so don’t get caught by it!
Oxidation of Secondary Alcohols
When it comes to the secondary alcohols, the reaction can only go through one round of oxidation. And yes, the ketone, just like the aldehyde, can easily form a hydrate here as well. However, since there’s no hydrogen on the carbon with the -OH in this case, we cannot oxidize it any further. And so, the molecule stays as the ketone.
Examples of the Jones Oxidation
Let’s now look at a few example of the Jones oxidation reaction.
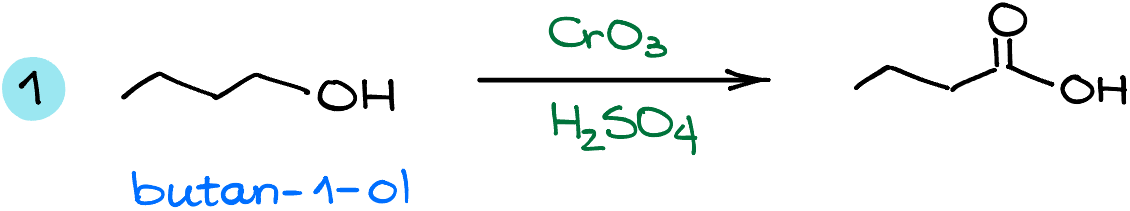
In the first example I have a simple butan-1-ol molecule as a starting material. This one gets oxidized into a corresponding butanoic acid. This is a fairly straightforward example.
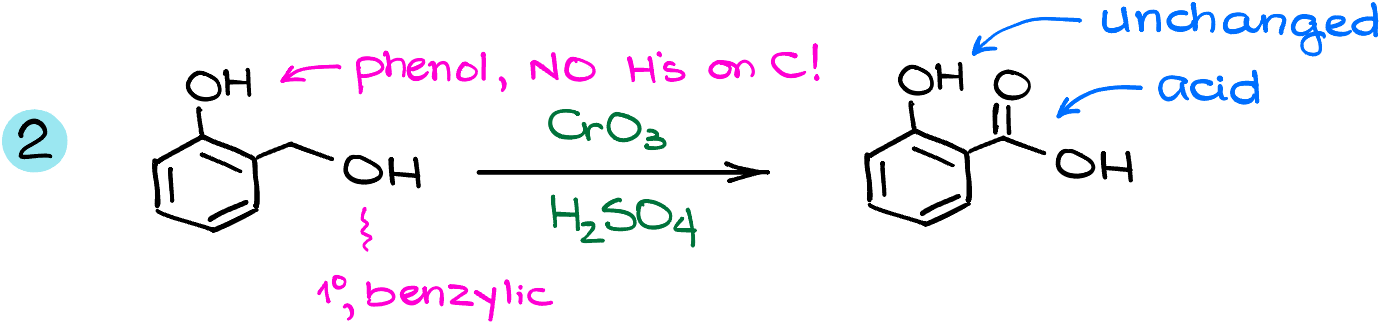
Next example features the phenolic -OH along the benzylic -OH. Since the carbon with the -OH on the aromatic ring doesn’t have any hydrogens on it, this carbon cannot be oxidized further, so we’ll keep it as it. However, the primary alcohol here will be oxidized all the way to the carboxylic acid.
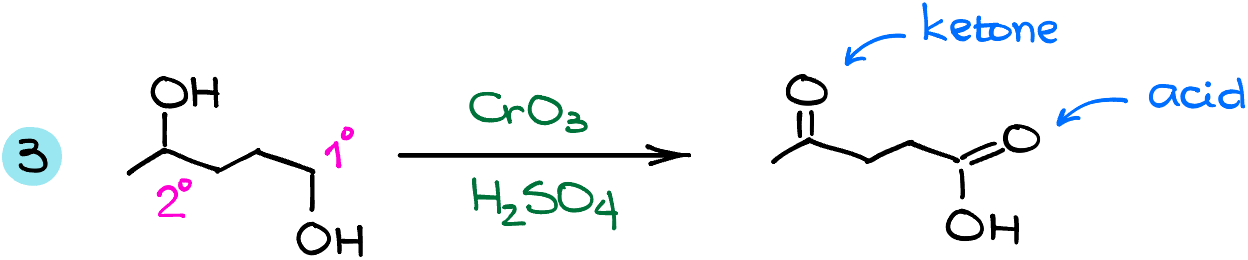
In this example, we see the secondary and the primary alcohol groups in the same molecule. Since both can undergo the oxidation, we’ll end up with a ketone from the secondary alcohol, and a carboxylic acid from the primary one.

Finally, in my last molecule, I have a secondary and a tertiary alcohol groups. In this case, we’ll keep the tertiary alcohol untouched since it cannot oxidize any further; and our secondary alcohol will give us the corresponding ketone functional group.
