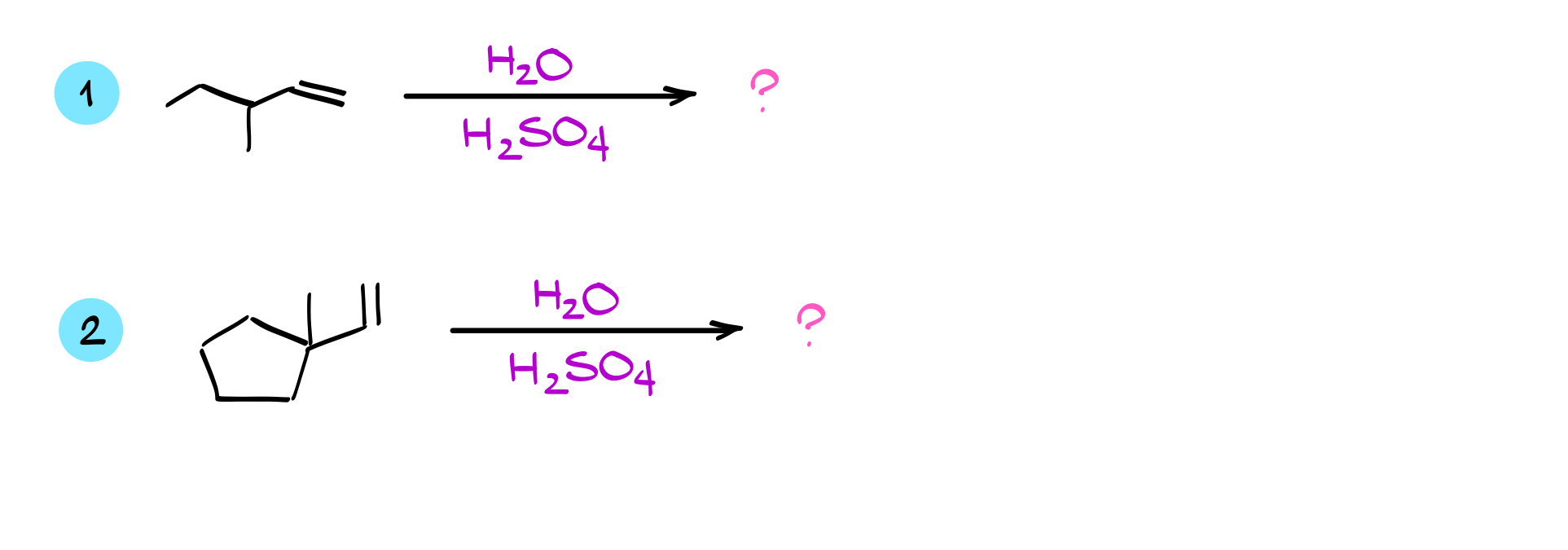Hydration of Alkenes
This tutorial covers the hydration of alkenes, a reaction that bears similarities to hydrohalogenation. Like hydrohalogenation, it follows the Markovnikov rule, forms a carbocation intermediate, and is prone to rearrangements. However, there are key differences that you must understand to avoid getting tricked on an exam.
General Scheme of the Alkene Hydration
In a typical hydration reaction, water is added across the double bond between carbon atoms. Importantly, this reaction requires a catalyst, usually a strong Brønsted acid such as sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄). The final product consists of one carbon gaining a hydrogen (just like in hydrohalogenation), while the other carbon receives an OH group.
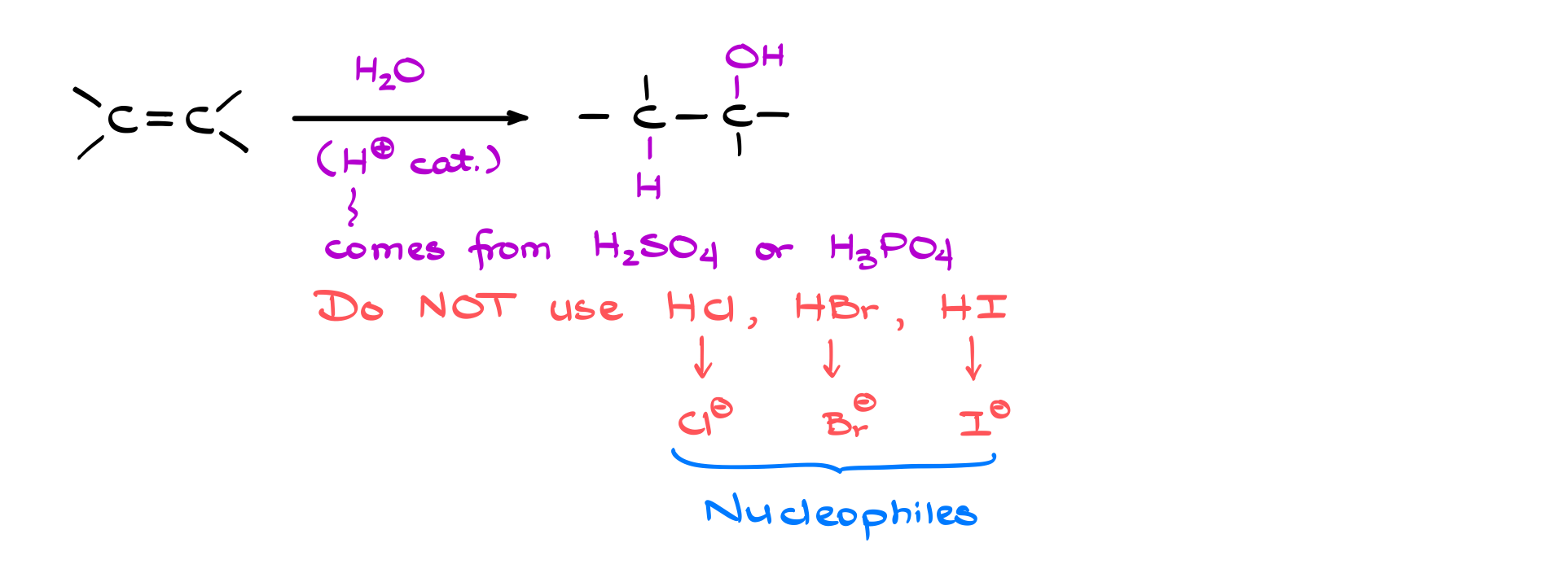
It’s crucial to never attempt this reaction using HCl, HBr, or HI as the acid catalyst. These hydrogen halides add directly across the double bond, consuming the catalyst entirely. The halide ions (Cl⁻, Br⁻, I⁻) are strong nucleophiles that would compete with water for nucleophilic attack, ultimately reacting with the carbocation instead and preventing the proper formation of the hydration product.
Hydration of Alkenes Mechanism
There are two main approaches to the reaction mechanism. The first suggests that H⁺ from H₂SO₄ directly adds across the double bond, forming a carbocation. While this method appears in some textbooks, especially in introductory organic chemistry courses, it is statistically unlikely and often considered incorrect by instructors. The second approach, which is more widely accepted, begins with the formation of an electrophile. Here, water reacts with sulfuric acid in a simple acid-base proton transfer, generating hydronium ion (H₃O⁺) and the conjugate base HSO₄⁻. This step is significantly more likely because H₃O⁺ is a much stronger acid and a more effective electrophile than water.

But why do we need a catalyst at all? Why can’t water just react with the double bond directly? The answer lies in its pKa value. Water has a pKa of around 14 (some textbooks list it as 15.7), meaning it is not acidic enough to supply a sufficient amount of H⁺ into the reaction system. In contrast, H₃O⁺ has a pKa of 0 or even -1.7 in some sources, making it a much stronger acid capable of effectively supplying protons.
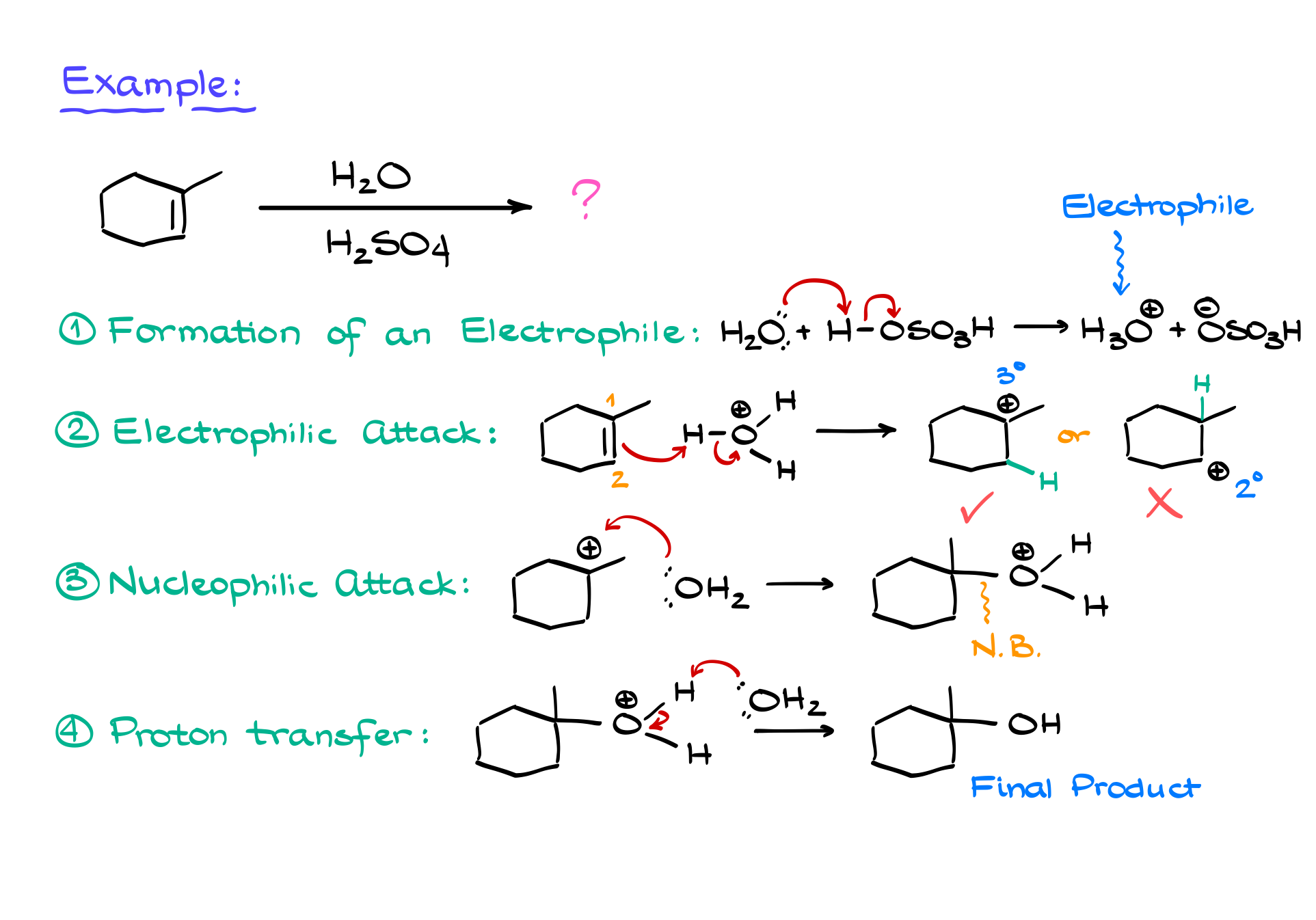
To see this mechanism in action, let’s examine a specific example. Suppose we are hydrating 1-methylcyclohexene. The first step is the formation of the electrophile, H₃O⁺, via the reaction between water and sulfuric acid. The next step is the electrophilic attack, where the double bond donates electrons to H₃O⁺, leading to the formation of a carbocation. Depending on which carbon the proton adds to, we could get different carbocation intermediates. However, following the Markovnikov rule, the more stable carbocation (in this case, a tertiary carbocation) will preferentially form over a less stable secondary carbocation.
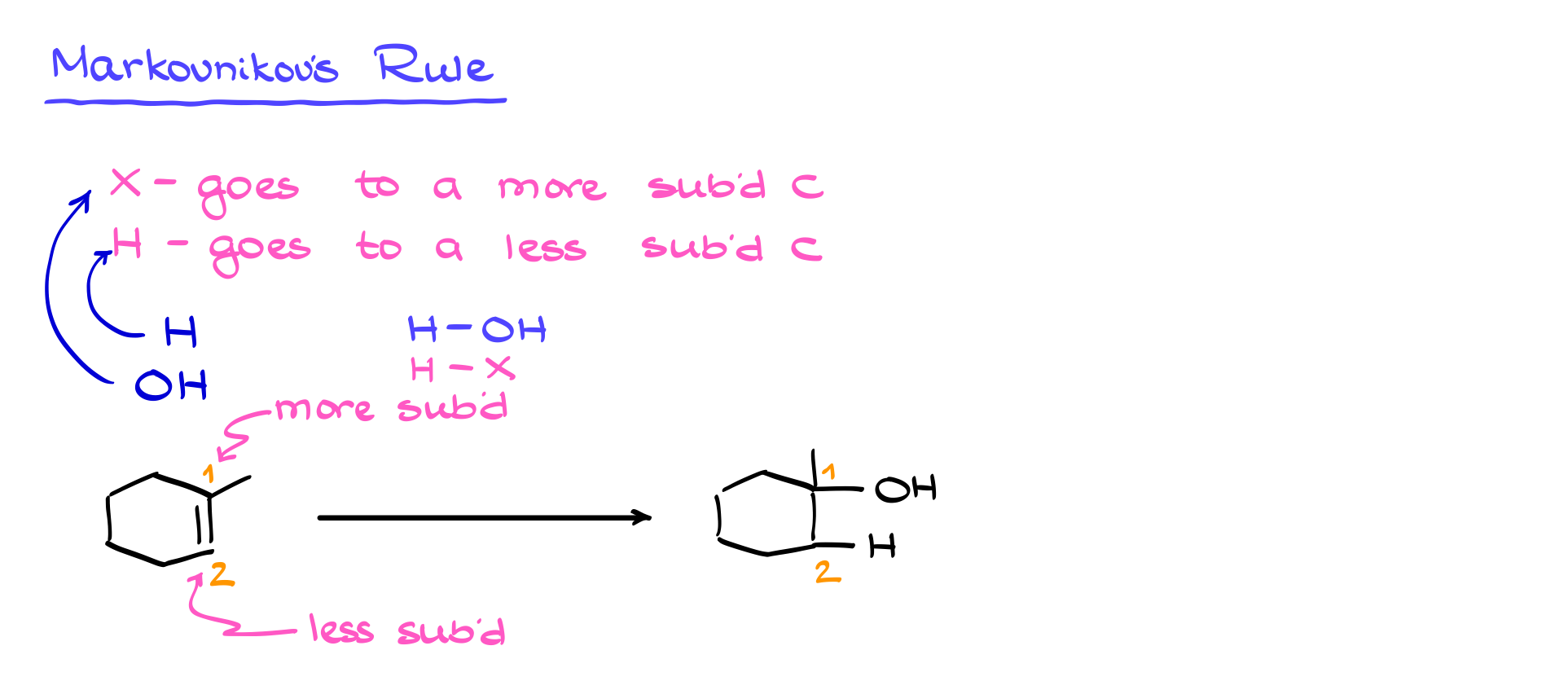
Once the carbocation is formed, the next step is the nucleophilic attack. Here, water, acting as a nucleophile, donates a lone pair of electrons to the positively charged carbon, forming a new C-O bond. This intermediate is still protonated, making it unstable, so the final step is deprotonation. Another water molecule (acting as a base) removes the excess proton, neutralizing the product and regenerating H₃O⁺, which can continue catalyzing further reactions.
Carbocation Rearrangements
Now, an important caution: this reaction is prone to carbocation rearrangements. If a more stable carbocation can form, the molecule will undergo a shift. In our example, if a secondary carbocation forms next to a tertiary position, a hydride shift (where a hydrogen with its bonding electrons moves) will occur to stabilize the intermediate. If no hydrogen is available, an alkyl shift may happen instead, moving an entire carbon-containing group. These shifts ensure that the most stable carbocation forms before the nucleophilic attack occurs.
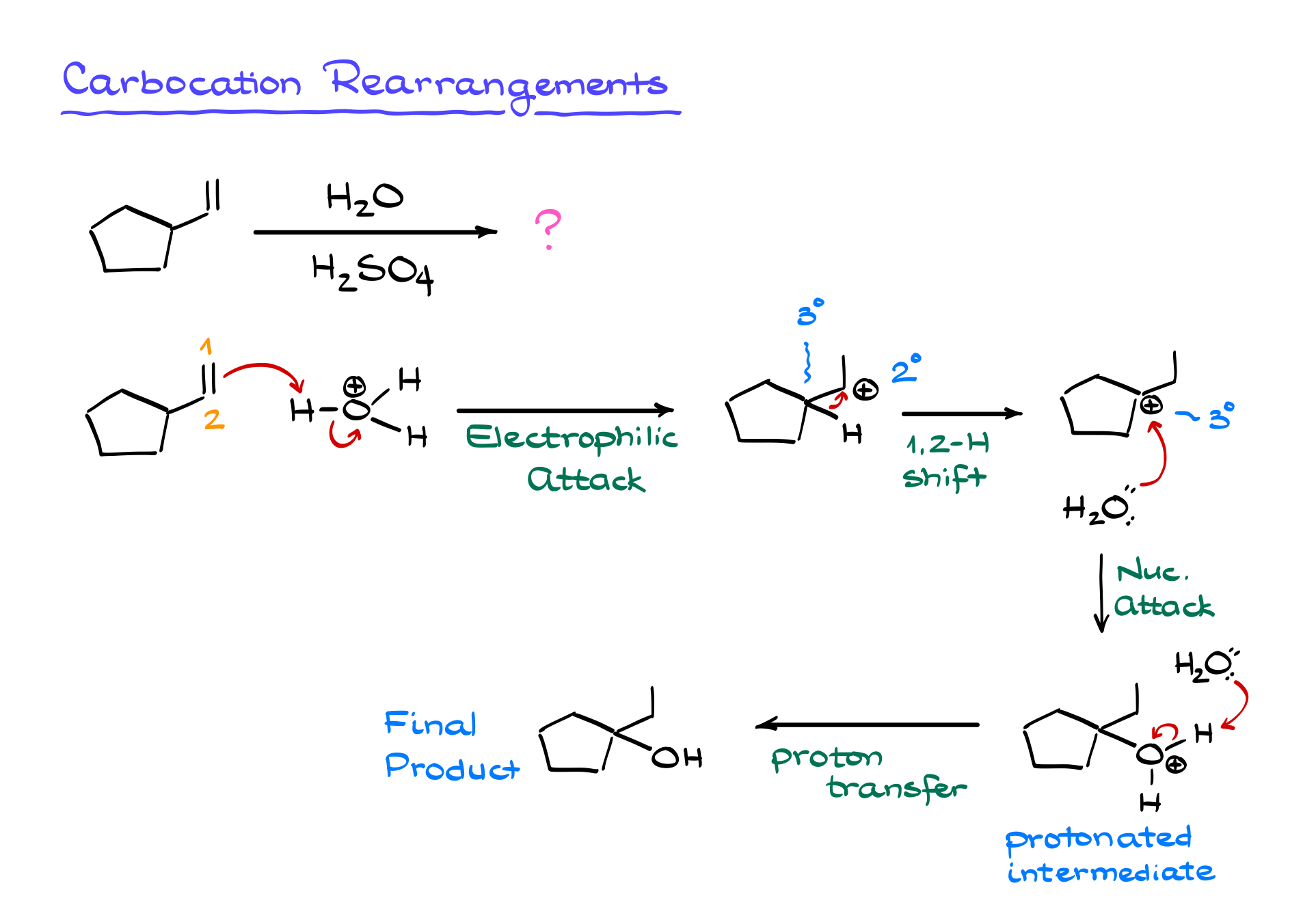
Because carbocation rearrangements are a common trap on exams, always check for them as soon as you generate a carbocation. Instructors love testing this concept, so be extra vigilant. If you don’t consider possible rearrangements, you could predict the wrong product, costing you points.
To reinforce your understanding, try applying this mechanism to a different reaction and see if you can correctly determine the major product. Once you’ve worked through it, double-check for carbocation rearrangements and compare your answer.
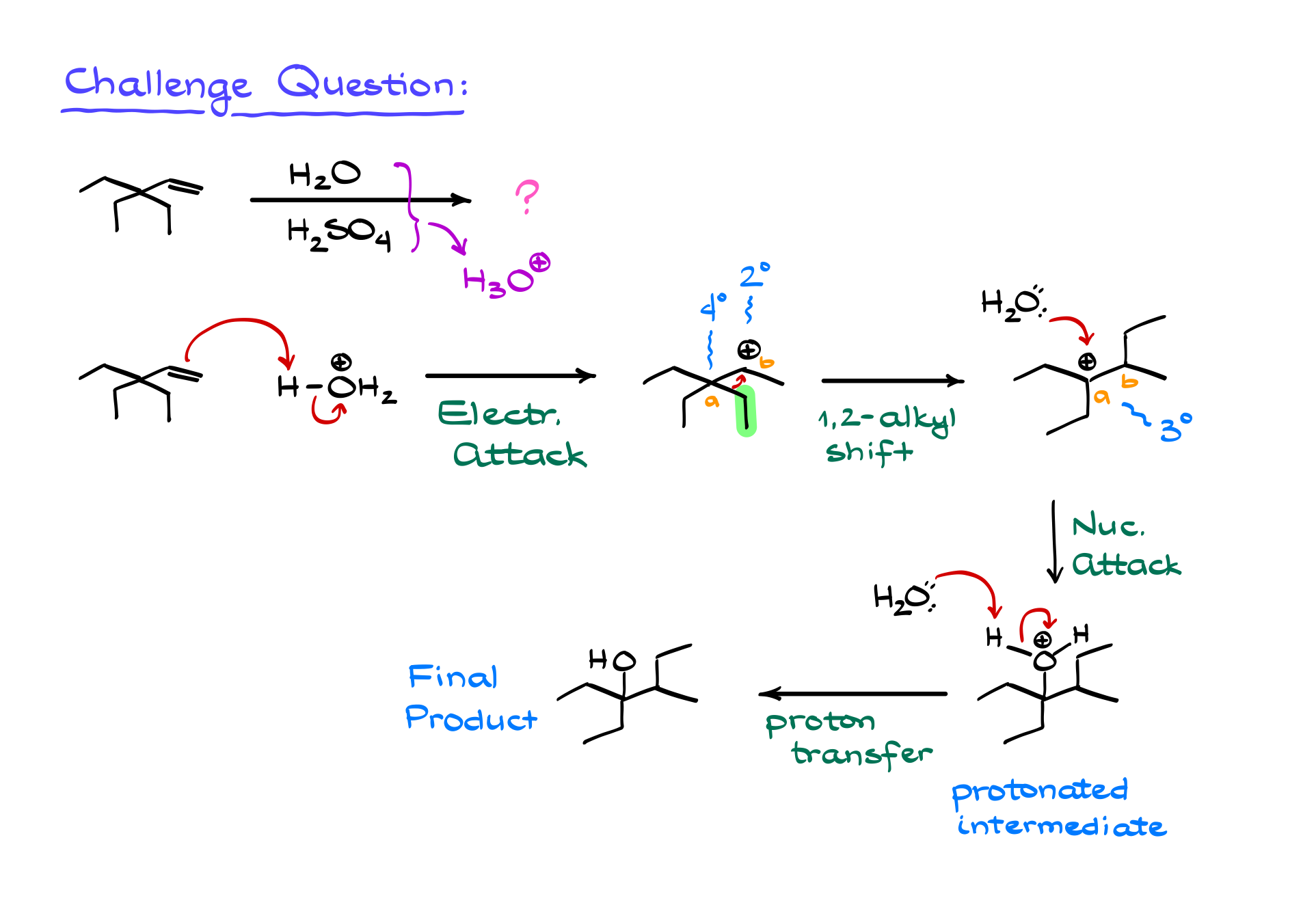
Hydration of alkenes is a fundamental reaction for synthesizing alcohols, making it a key reaction in multi-step synthesis problems. You’ll encounter it repeatedly throughout your organic chemistry course, so mastering it now will pay off later. Just remember: always check for carbocation rearrangements, and you’ll be on the right track.
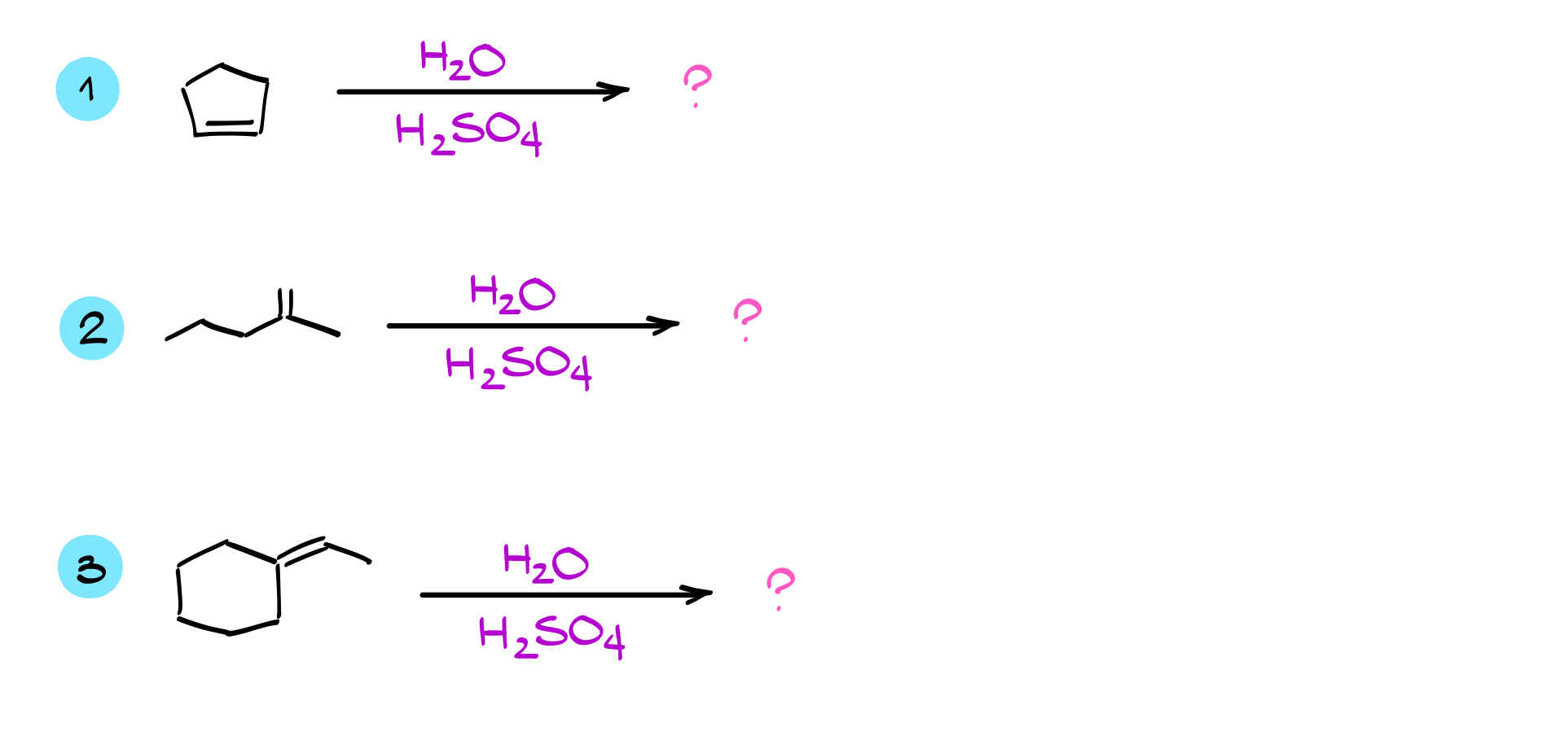
Practice Quiestions
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!