How to Use Curved Arrows
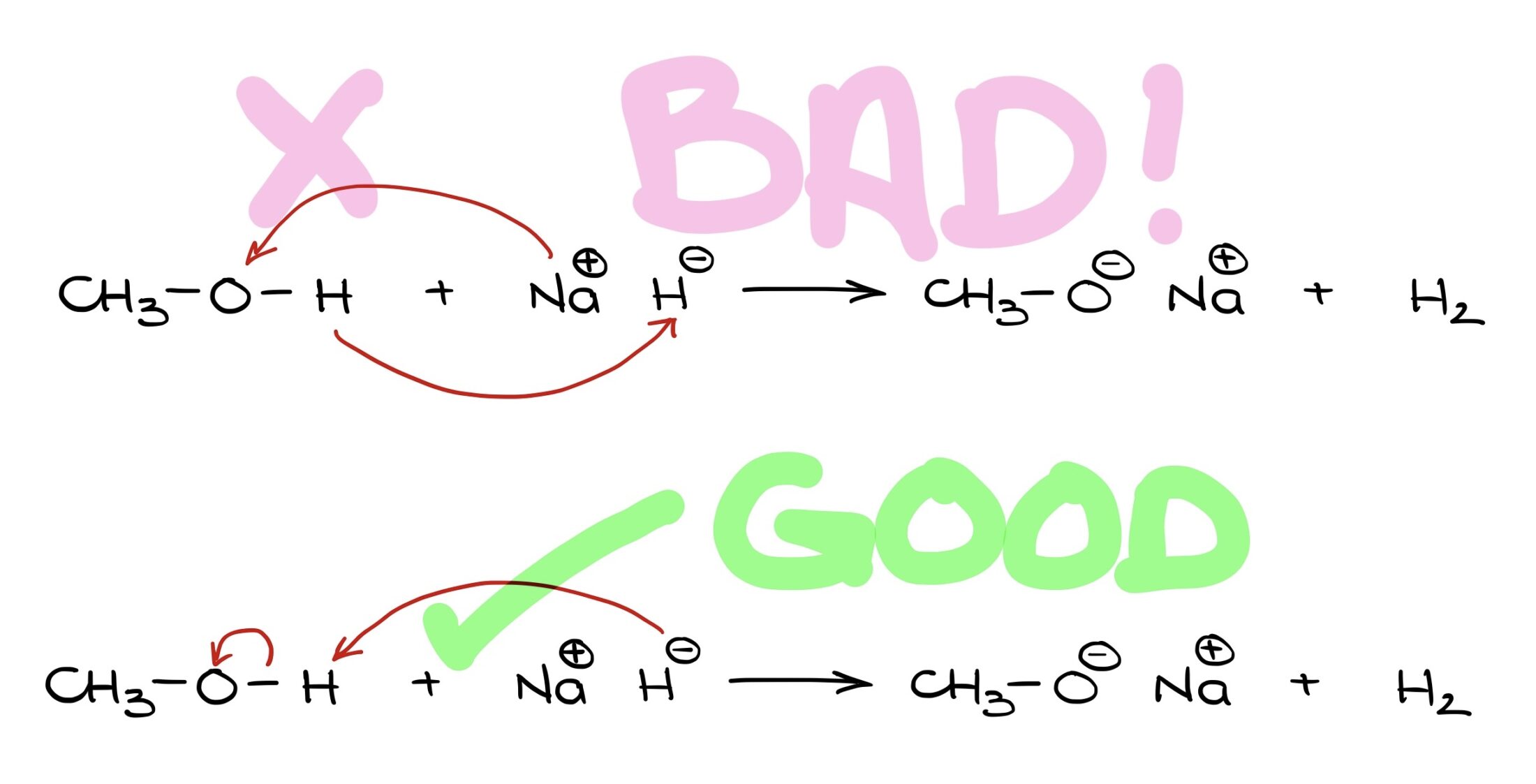
Why are curved arrows in the first example unsatisfactory, but great in the second? Let’s explore this. Hello, I’m Victor, and today we’re diving into the use of curved arrows in organic chemistry.
There are three primary scenarios where we use these arrows:
- In resonance, indicating the movement of electrons between resonance contributors.
- Demonstrating the movement of electron density in acid-base chemistry.
- Displaying electron density movement in various reactions.
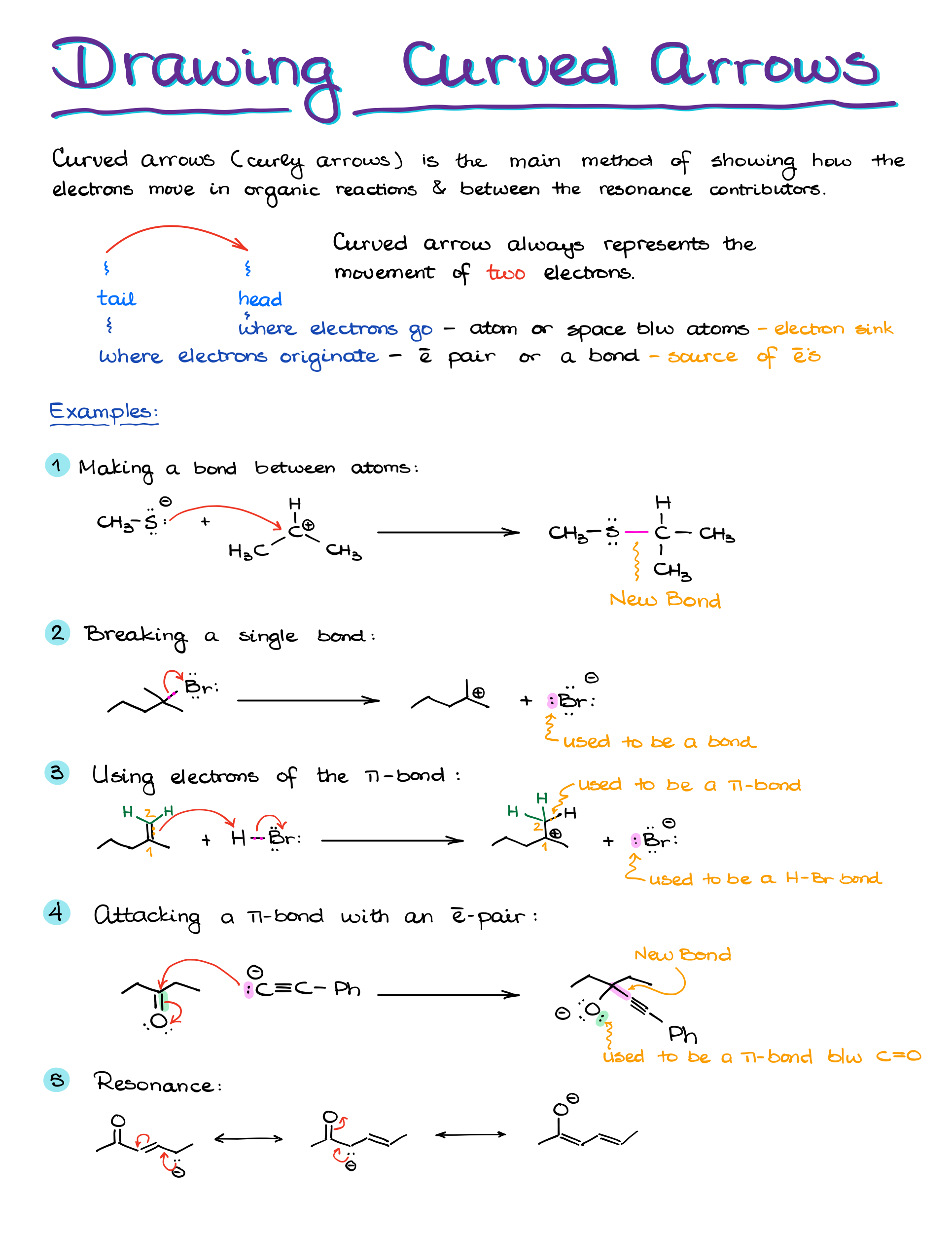
“Anatomy” of a Curved Arrow
Before discussing the curved arrows’ functionality, let’s understand their structure. A curved arrow has two key points:
- The origin, or the source of electron density, known as the tail.
- The destination, where electrons are headed, represented by the arrowhead.
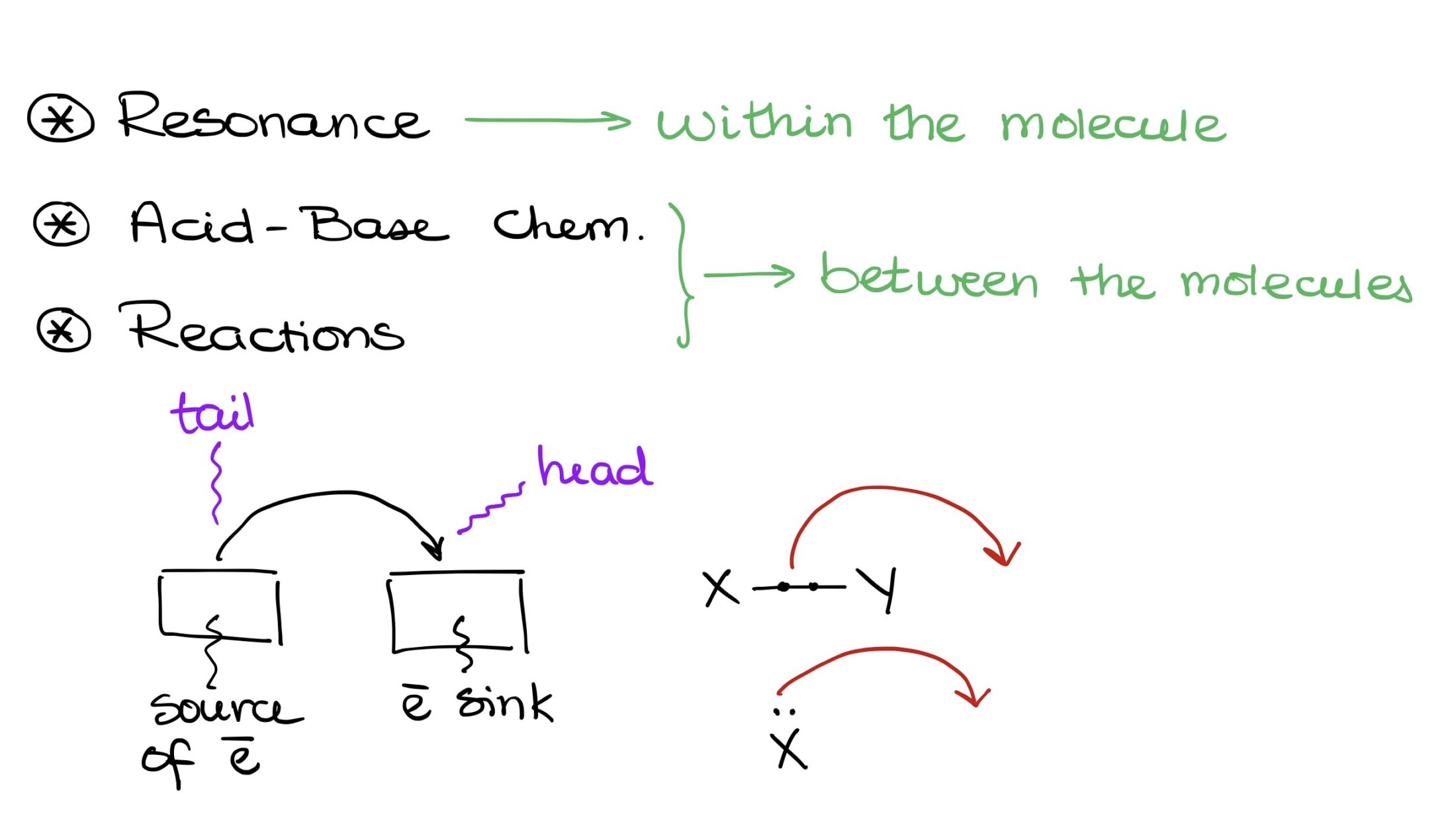
Electrons start at the tail and move towards the arrowhead. They either exist between two elements, say X and Y, or as an electron pair on a single element.
Examples of Curved Arrows in Organic Chemistry
Take this reaction, for instance: moving a proton from HCl to H2O to form H3O. The electron pair from oxygen is shared with hydrogen. At the same time, electron density shifts from the hydrogen-chlorine bond to chlorine.
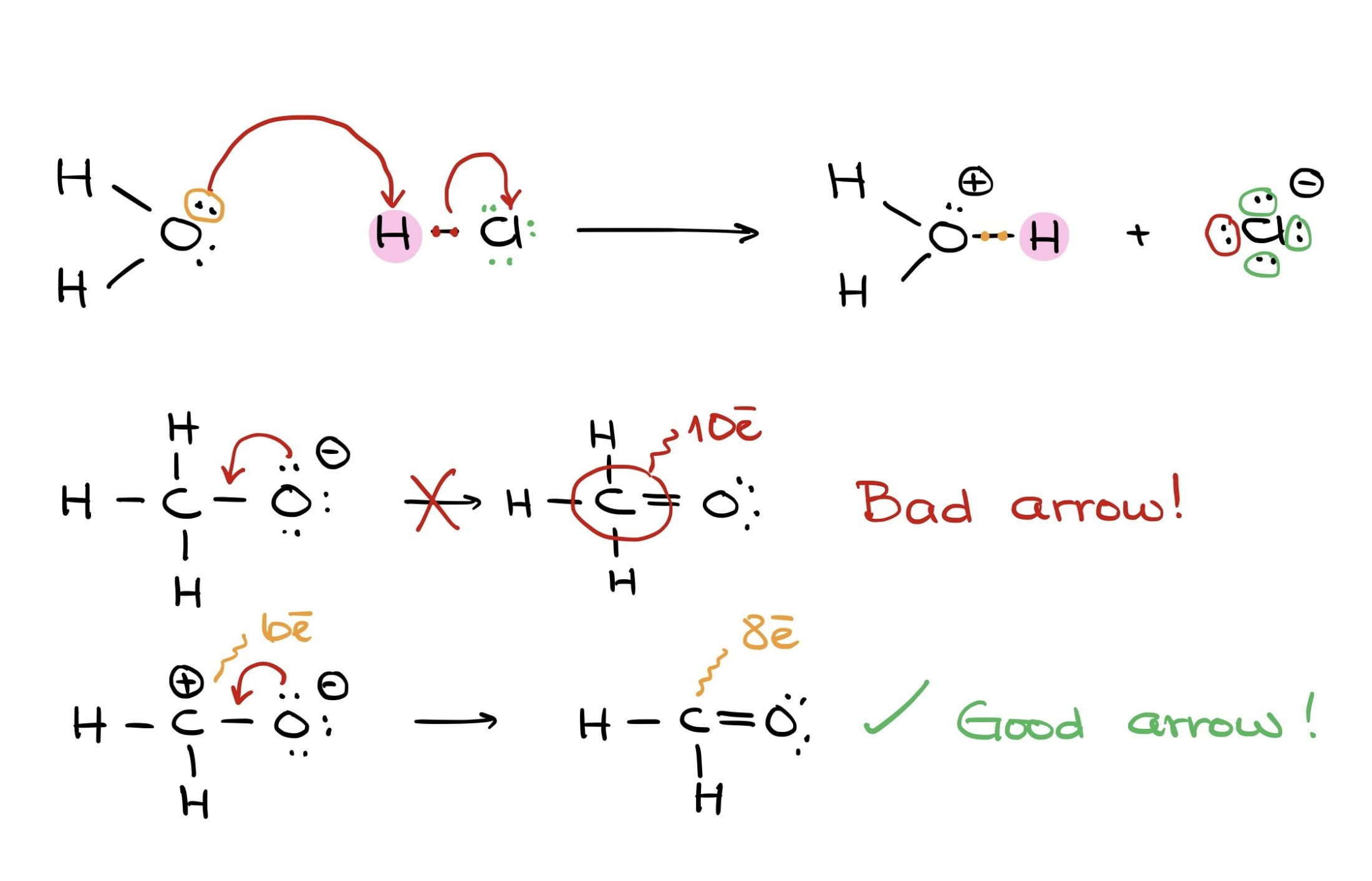
Remember, if electrons are pushed to an atom, that atom must either accept them or break a bond to accommodate them.
For example, pushing an electron pair from oxygen to carbon in a methoxide anion can be problematic because the carbon ends up exceeding its octet. But, pushing electrons onto a carbocation with an empty orbital can result in a satisfactory carbon-oxygen double bond.
If we have a molecule with carbon bonded to oxygen, hydrogen, and chlorine, pushing electrons only to carbon results in an unsatisfactory structure with 10 electrons around carbon. But, simultaneously pushing electrons to carbon while breaking its bond with chlorine yields a correct structure.
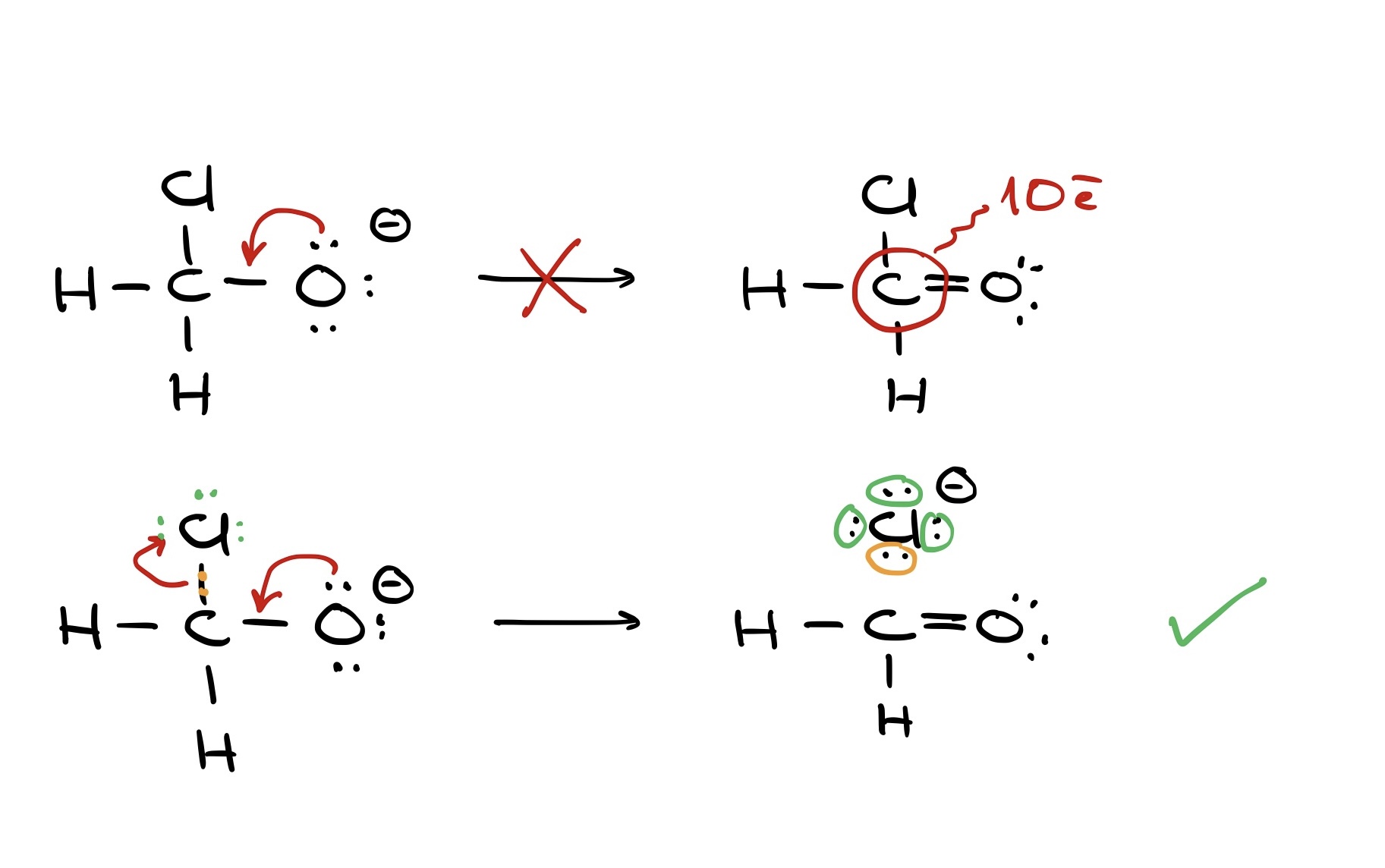
Using curved arrows effectively in organic chemistry means always showing electron movement, not atom movement. The electrons guide the atoms.
For success with curved arrows:
- Start arrows from a bond or an electron pair.
- Aim the arrow to the electron destination, either back to an atom or forming a new bond.
- Always depict electron movement, not atom movement.

Practice Questions:
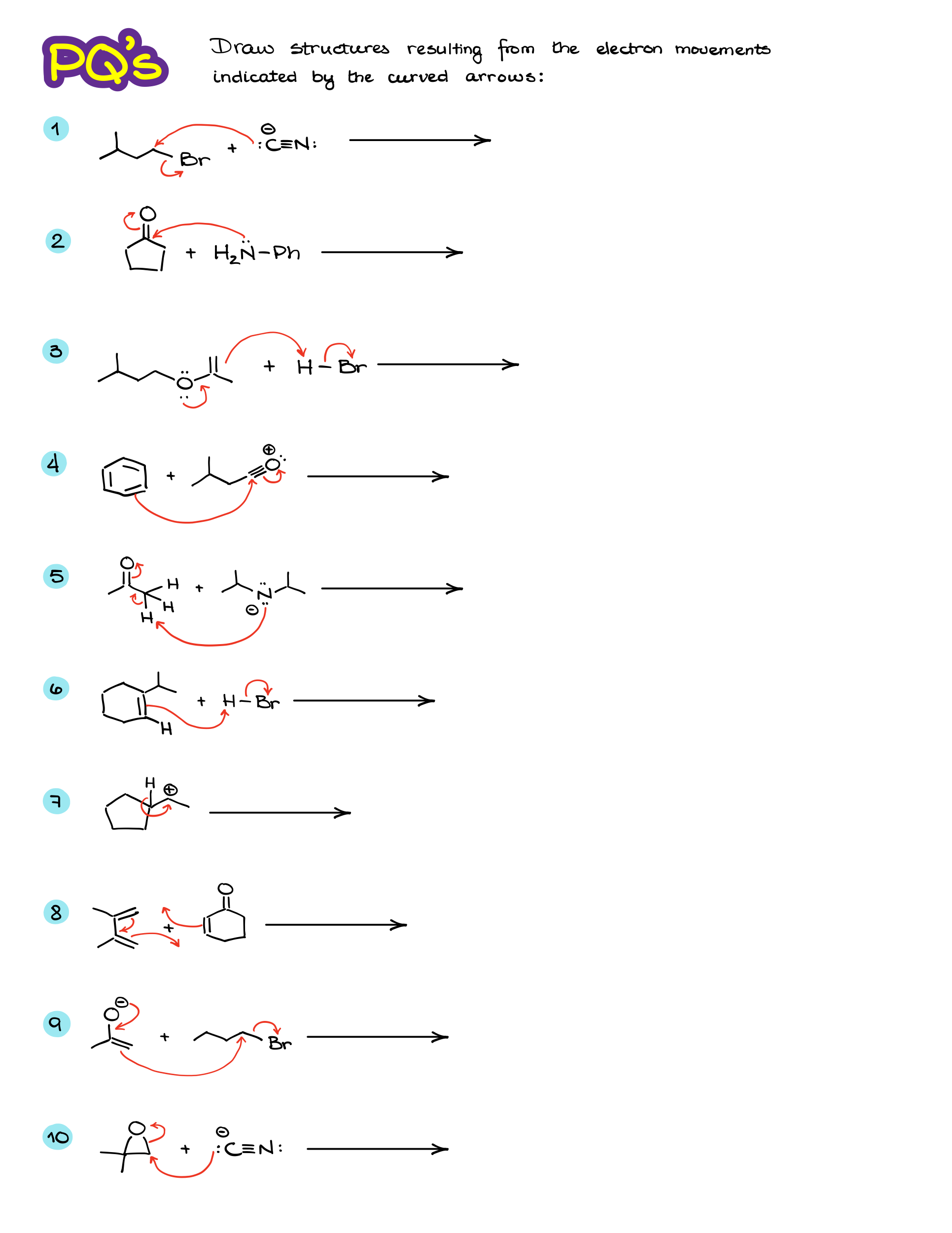
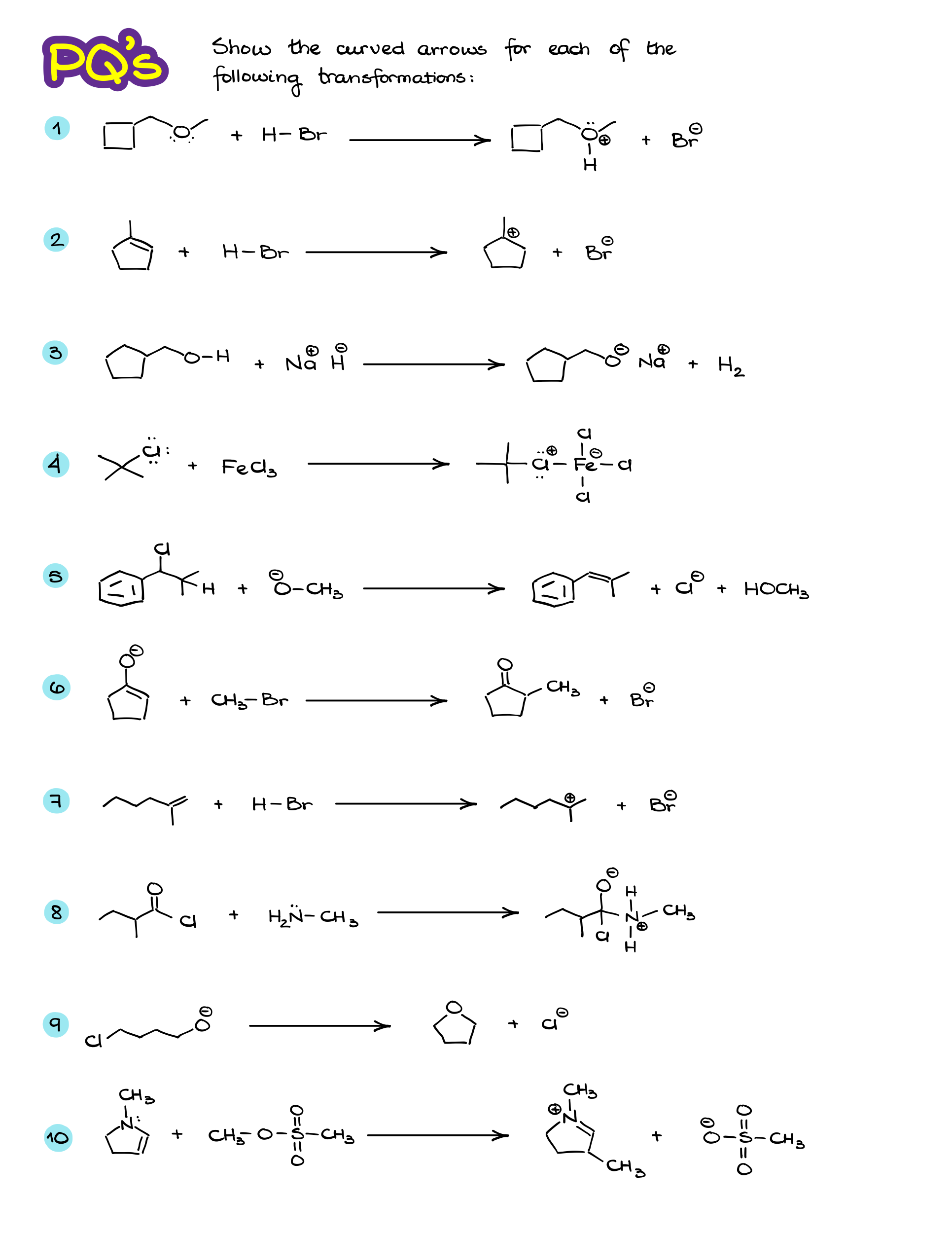
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!
