How To Predict the Diels-Alder Starting Materials
If thinking about synthesizing molecules like this via the Diels-Alder reaction makes you dizzy, you are not alone. So give me a few minutes, and I’ll show you how to easily predict the Diels-Alder reactants.

Understanding the Diels-Alder Reaction
Let’s start by looking at the Diels-Alder reaction itself.
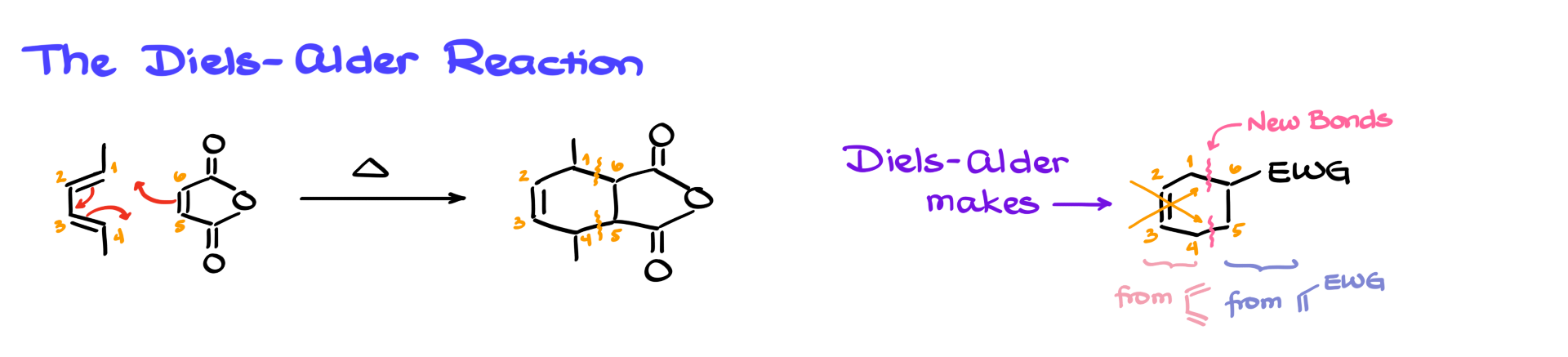
Suppose we have two molecules reacting with each other, and as a result, we move our electrons in a circular fashion. If we number the atoms as 1, 2, 3, 4, 5, and 6, we will form new bonds between carbons 4 and 5 and between carbons 1 and 6. Additionally, a double bond will appear between carbons 2 and 3. The final product is a six-membered ring, where the newly created bonds are positioned opposite the double bond.
The key takeaway here is that the Diels-Alder reaction always forms a six-membered ring with a double bond. Sometimes, if the dienophile contains a triple bond, two double bonds will appear in the product, but typically, we expect one double bond with possible electron-withdrawing groups attached. The new bonds always form across from the double bond in the ring, making the position of the double bond a useful guide for determining where bonds will form.
Another important aspect is how we can trace the origin of each atom in the product. Atoms 1 through 4 always come from the diene, while atoms 5 and 6 come from the dienophile.
Predicting the Starting Materials
Let’s apply this approach to a real example.

If we are given a six-membered ring with a double bond, we start our analysis by numbering the double bond’s atoms as 2 and 3. Then, we number the rest of the ring and mark the new bonds that were formed—between 1 and 6 and between 4 and 5.
Since atoms 1 through 4 originated from the diene and atoms 5 and 6 from the dienophile, we can break the molecule at these points and reconstruct the starting materials. The diene must contain two double bonds, whereas the dienophile must have a C=C or C≡C bond, often with electron-withdrawing group(s).
However, molecules are not always presented in the most convenient way.
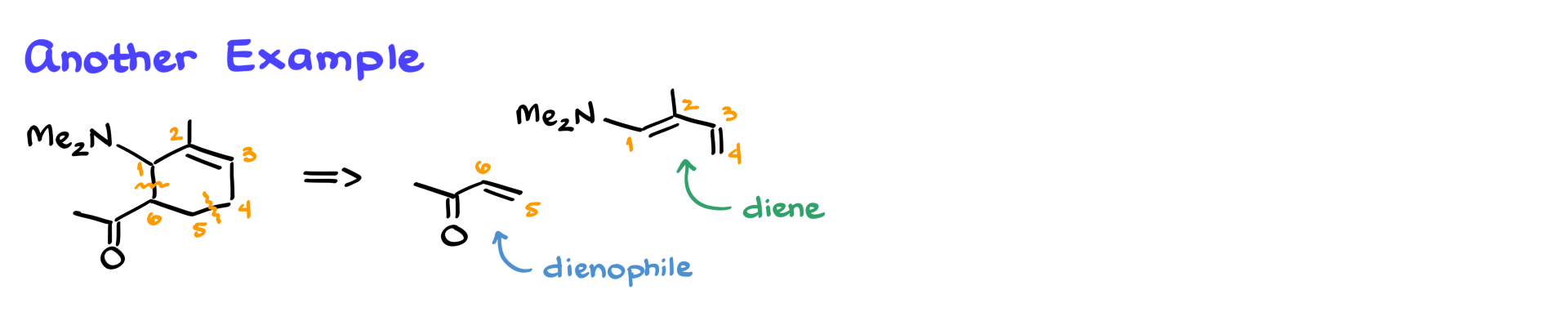
If the double bond appears in an unusual position, we still follow the same steps: number the atoms, identify the new bonds, and split the structure accordingly. Even if the molecule is rotated or drawn in a different orientation, the same principles apply.
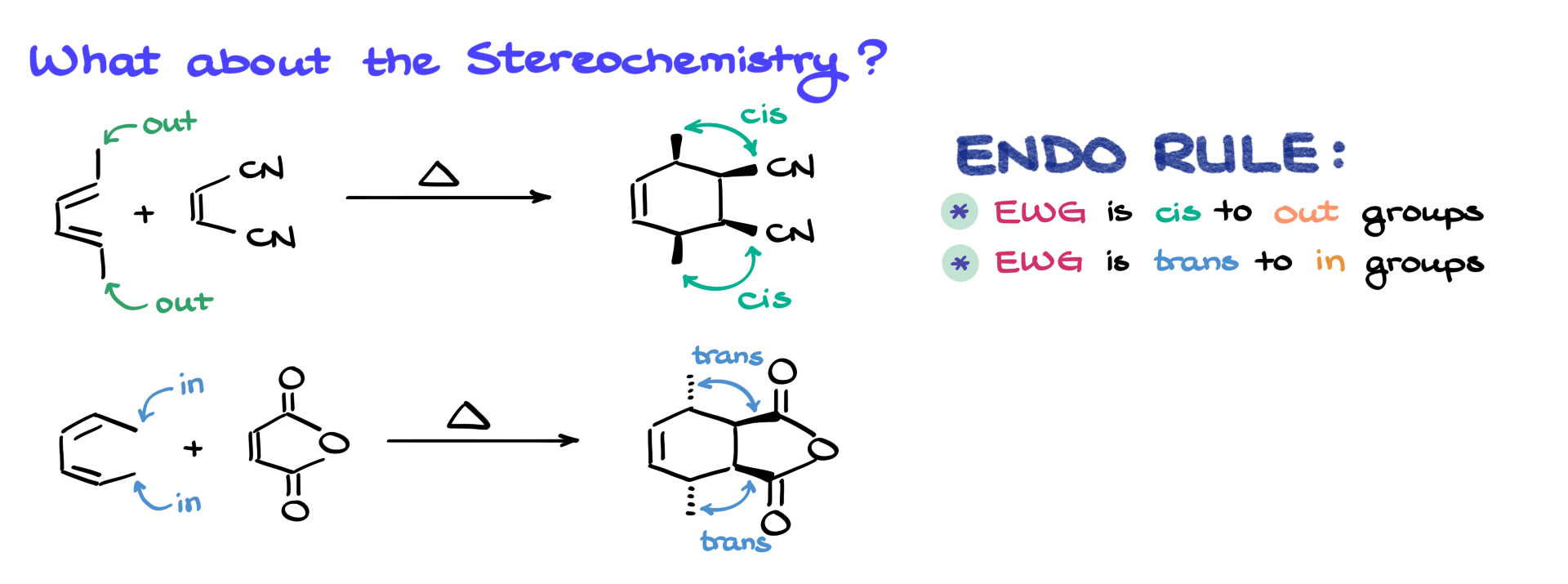
Considering Stereochemistry
Stereochemistry plays a crucial role in the Diels-Alder reaction. The endo rule shortcut is that electron-withdrawing groups (EWGs) on the dienophile will always end up cis to the diene’s substituents on atoms 1 and 4 in the final product.
Let’s apply this to a real case.
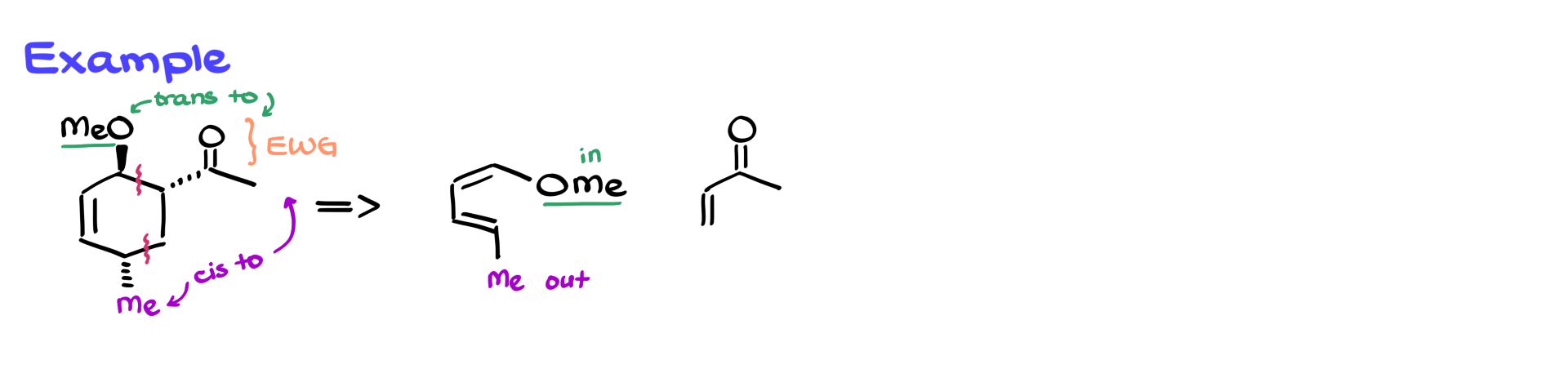
Suppose we need to determine the starting materials for a given product (picture above). The first step is recognizing that the carbonyl group (C=O) in the product is an electron-withdrawing group. By identifying where new bonds formed and checking which groups are cis or trans to the EWG, we can determine whether each group in the starting material was an IN or OUT group.
If stereochemistry is specified, you must consider it when drawing the starting materials. If your instructor does not explicitly state that stereochemistry can be ignored, failing to account for it can lead to incorrect answers.
Applying the Algorithm to Complex Molecules
Now, let’s return to the complex molecule from the beginning of this tutorial.
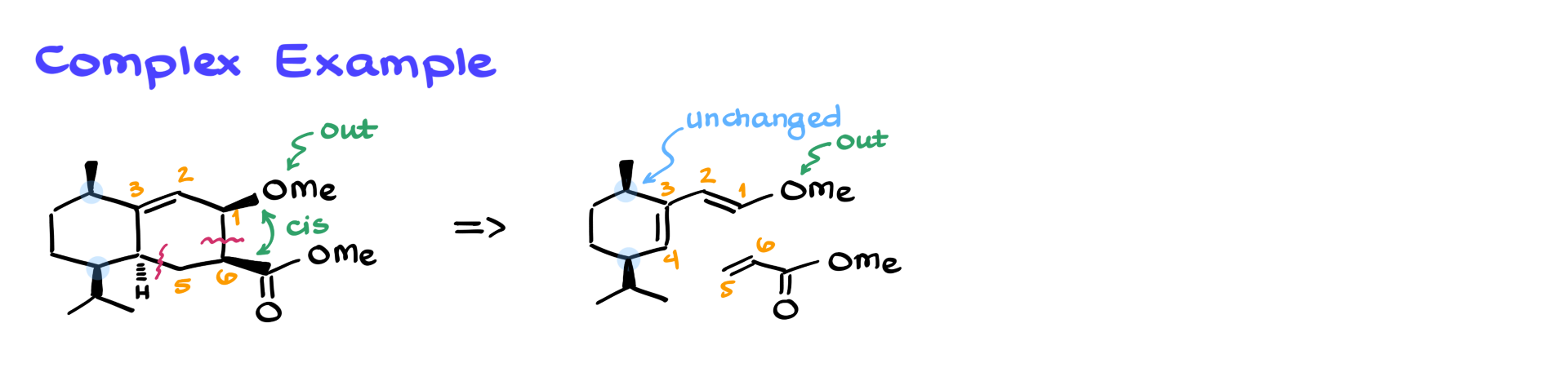
Although it might look intimidating, if we apply our algorithm step by step, predicting the starting materials becomes straightforward.
We start by numbering the double bond (carbons 2 and 3), numbering the remaining ring atoms, and marking the newly formed bonds (1-6 and 4-5). This immediately tells us how to break the structure into its original diene and dienophile components.
Adding stereochemistry makes things even more interesting. Suppose all bonds in a given product are cis. We first identify which part of the molecule corresponds to the electron-withdrawing group and then determine which groups were IN and which were OUT in the starting materials. The IN and OUT positions are preserved in the reaction, so this helps reconstruct the diene’s orientation.
Alternatively, if the product has mixed wedge and dash bonds, we analyze the relationship between the groups and the electron-withdrawing group to determine whether they were cis or trans in the starting materials.
Intramolecular Diels-Alder Reactions
No discussion of the Diels-Alder reaction would be complete without mentioning the intramolecular Diels-Alder reaction—the ultimate test of an organic chemistry student’s patience!
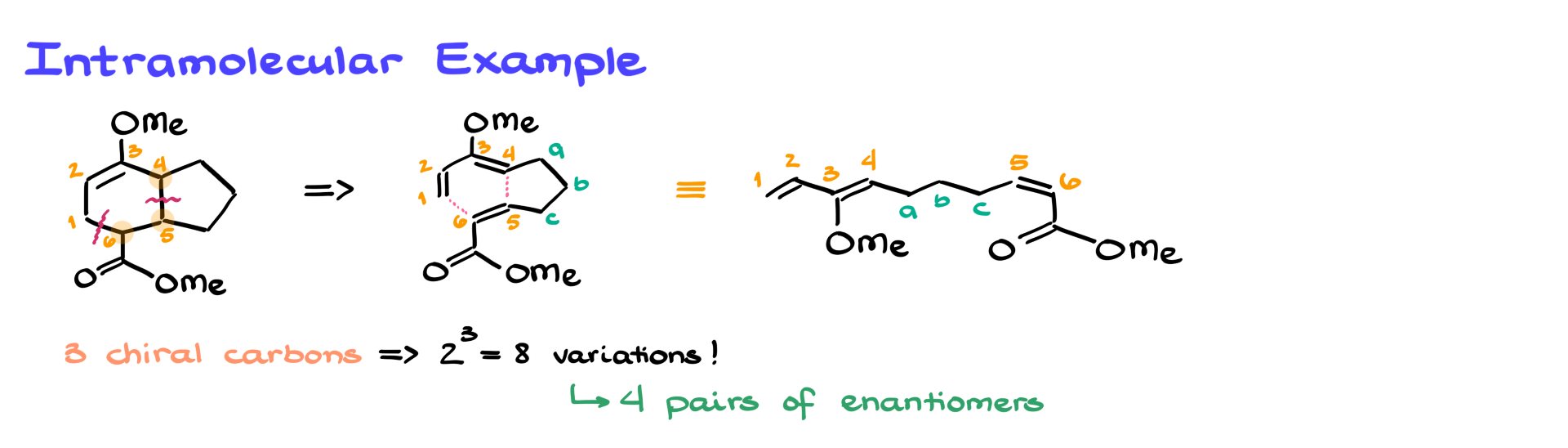
In an intramolecular reaction, the diene and dienophile are part of the same molecule, leading to bicyclic structures. The key to analyzing these reactions is the same:
- Identify the double bond and number the atoms.
- Mark the newly formed bonds in the product.
- Recognize that the molecule did not form two separate pieces but instead folded onto itself, creating a bicyclic system.
For example, if we analyze a bicyclic product above, we can see that atoms 1 through 4 belong to the diene and atoms 5 and 6 to the dienophile.
To make things more fun, let’s consider stereochemistry. Since an intramolecular Diels-Alder reaction I am using here as an example creates three chiral centers, we can theoretically get eight possible stereoisomers (or four pairs of enantiomers).
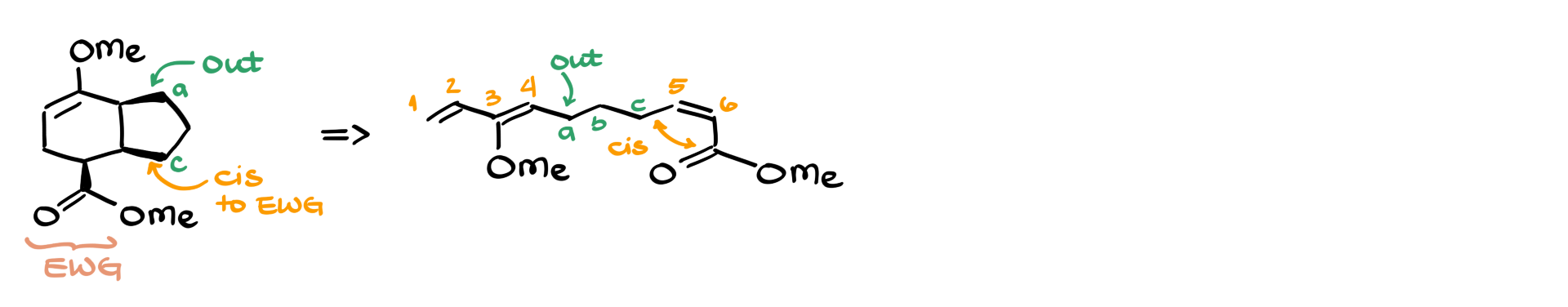
Let’s say we have a product where all bonds are cis. The first step is identifying the electron-withdrawing group. If a part of the diene is cis to the EWG, we mark it as OUT in the starting material, ensuring that this stereochemical relationship carries over to the product.
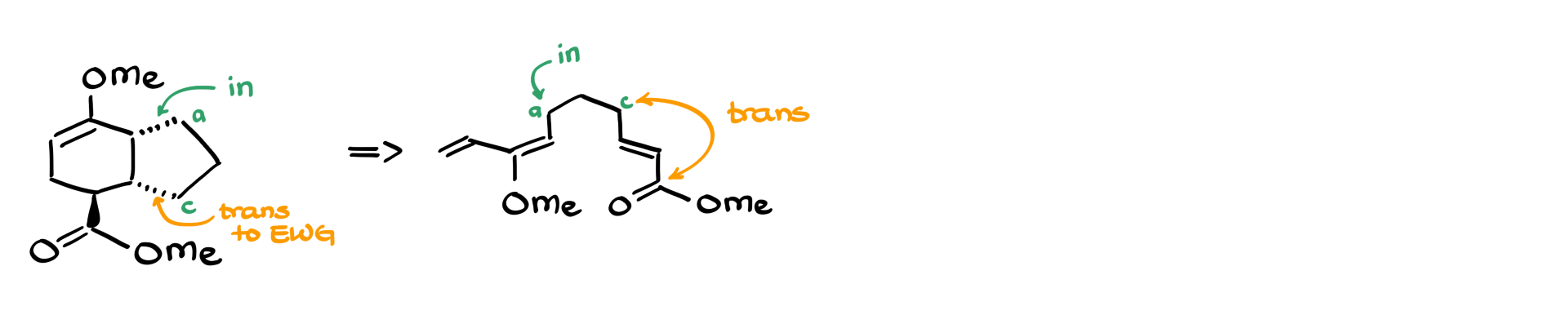
If we instead have a product where one part of the molecule is trans to the EWG, we adjust our starting material accordingly. This means recognizing which atoms were IN and which were OUT during the reaction.
Final Thoughts
The Diels-Alder reaction might seem intimidating at first, but using this structured approach, you can predict the starting materials for even the most complex products.
- Identify the double bond in the product.
- Number the atoms in the six-membered ring.
- Mark the newly formed bonds to see where the molecule split.
- Reconstruct the diene and dienophile, ensuring the correct number of double bonds.
- If stereochemistry is involved, apply the IN/OUT rule.
And, of course, let’s not forget the intramolecular Diels-Alder reaction, which takes all these concepts to another level by forming bicyclic structures with multiple stereocenters.
So, was this fun for you too, or am I just projecting my love for the Diels-Alder reaction onto you?
