How to Identify Chiral Atoms, Chiral Molecules, and Meso Compounds
In this tutorial, we’ll delve deep into the fascinating world of chirality in chemistry. We’ll cover not only what makes an atom or molecule chiral, but also discuss a topic that often confuses students—meso compounds. So, let’s get started!
What is Chirality?
First things first, what is a chiral object? A chiral object has a non-superimposable mirror image. This can apply to both macro objects like garden snail shells or your hands, and to micro objects like molecules or even individual atoms. For example, (R)-2-butanol and (S)-2-butanol are mirror images of each other and are non-superimposable. To understand more about R and S stereodescriptors and the Cahn–Ingold–Prelog (CIP) rules, you can check out this tutorial.

Chiral Atom vs. Chiral Molecule
It’s crucial to differentiate between a chiral atom and a chiral molecule. While the term ‘chiral’ can describe a molecule, it can also describe an atom within a molecule. The entire molecule can be chiral, or just a single atom in that molecule can display chirality.
Identifying a Chiral Atom
A chiral atom is an sp3-hybridized atom that has four different groups attached to it. This definition isn’t limited to carbon atoms; other atoms like nitrogen, sulfur, and phosphorus can be chiral as well.
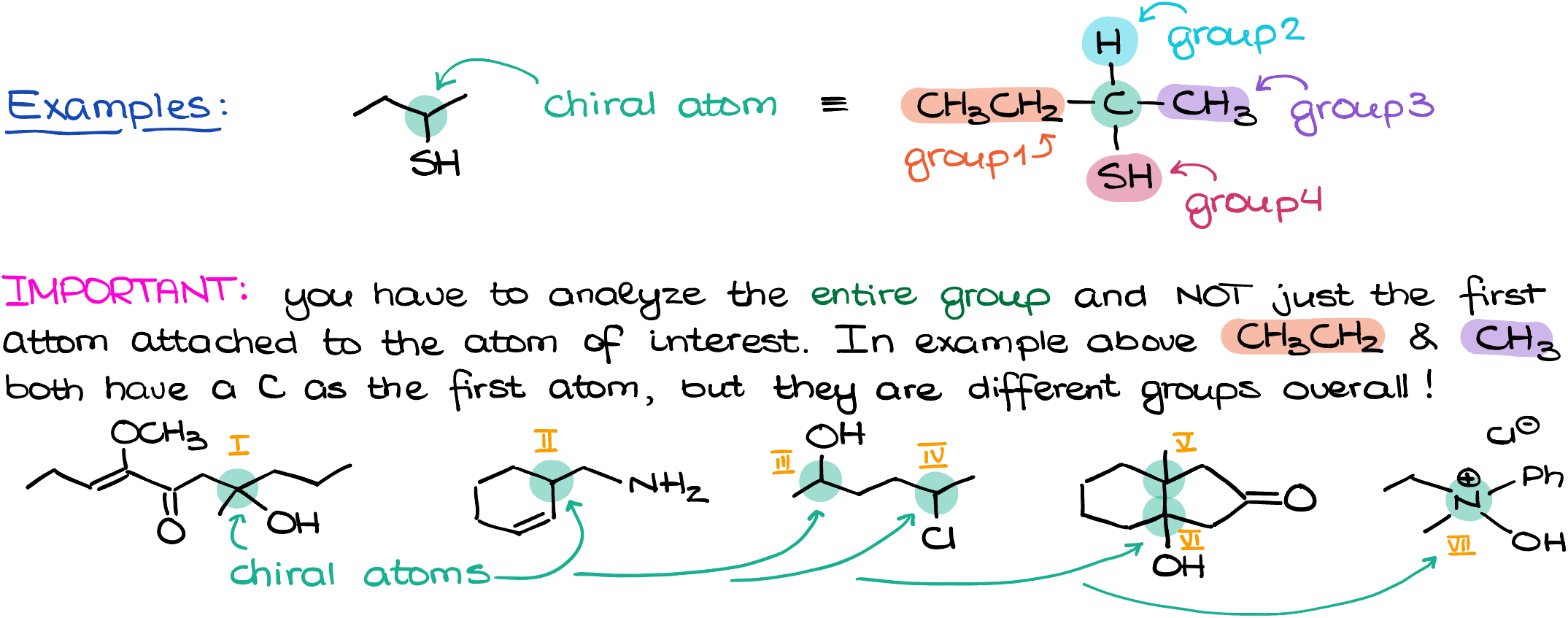
Analyzing the Entire Group
When identifying chiral atoms, always consider the entire group attached to the atom, not just the first atom in that group. For example, if you have an ethyl and a methyl group attached to a carbon atom, both groups may start with a carbon atom, but they are different groups entirely.
Consider Implicit Hydrogens
Implicit hydrogens might not be shown in the molecular structure, but they are still present and should be counted as a group when determining chirality. If a molecule is part of a cycle or chain, continue analyzing until you find a difference or the chain ends.
The Enigma of Meso-Compounds
A common misconception is that the presence of chiral atoms automatically makes a molecule chiral. However, this isn’t always the case. Meso-compounds are molecules that have chiral atoms yet are superimposable with their mirror images, making them achiral.
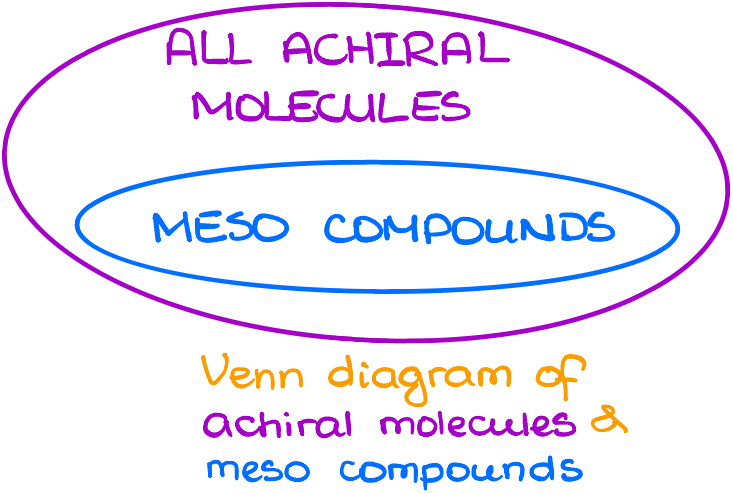
Characteristics of Meso-Compounds
Meso-compounds are always achiral, but not all achiral molecules are meso-compounds. A meso-compound is a subtype of achiral molecules, and it’s essential to understand that ‘meso’ describes the molecule, not the relationship between two molecules.
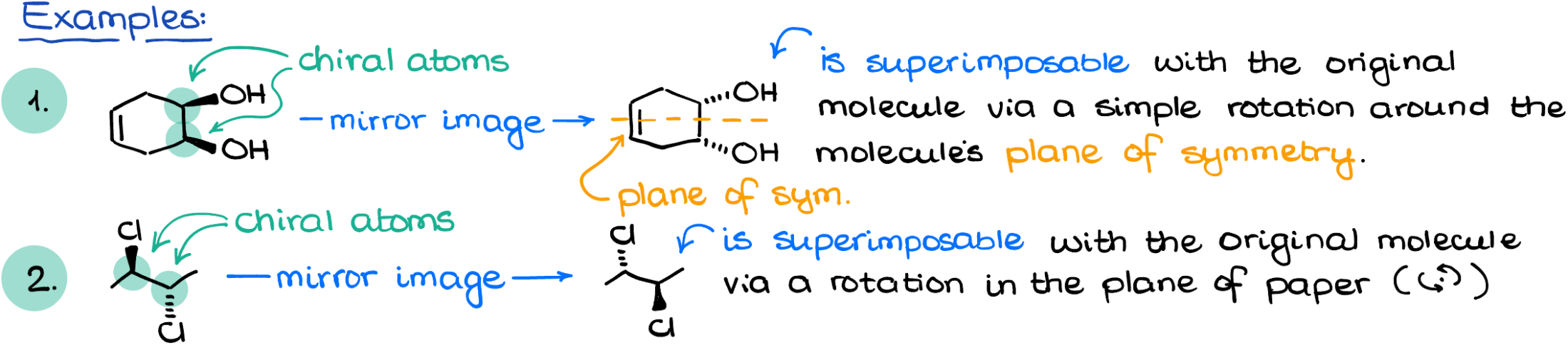
Planes of Symmetry
Another point to consider is planes of symmetry. A molecule with a plane of symmetry will look like a butterfly or an inkblot, indicating that it’s achiral even if it contains chiral atoms.

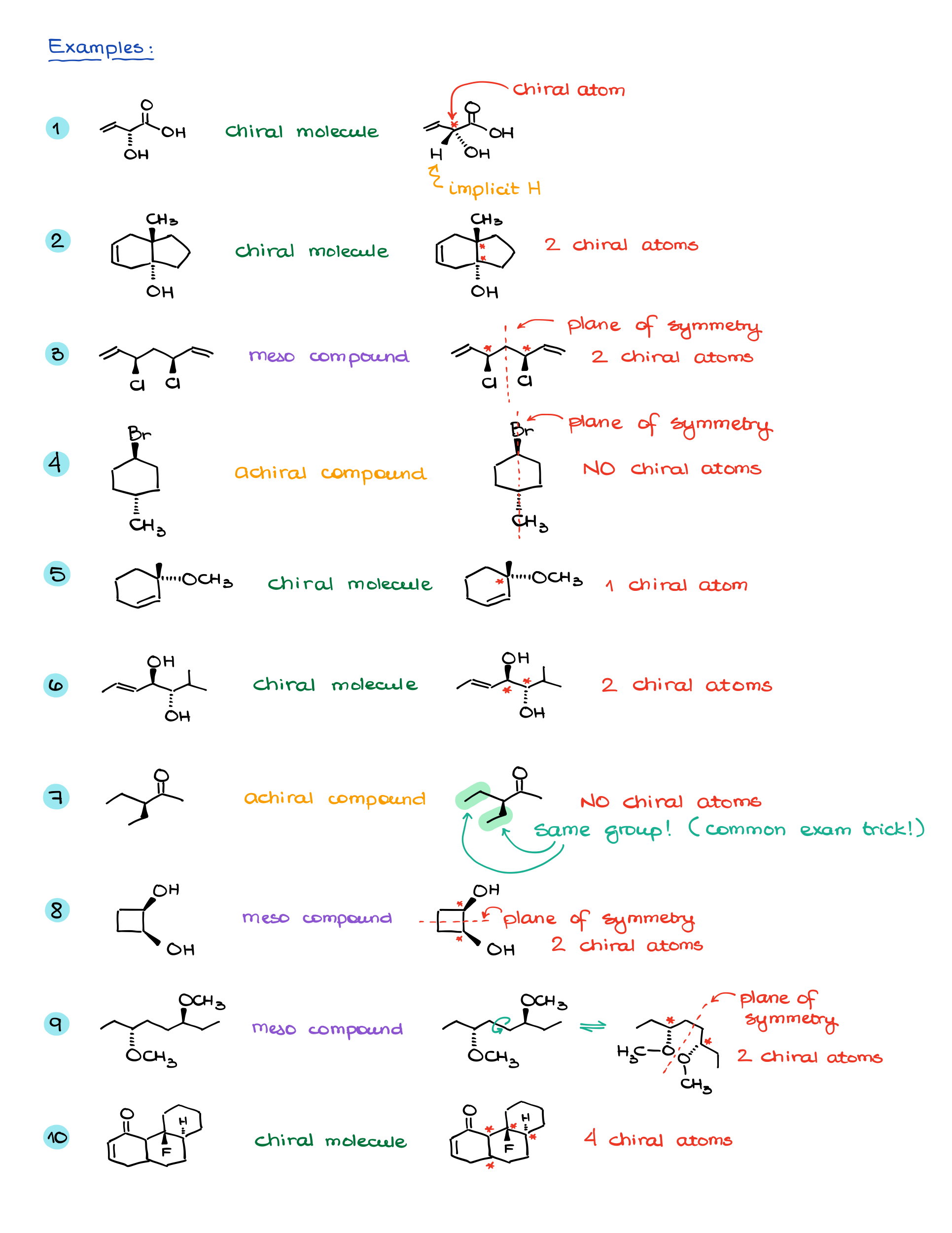
Examples and Practice
While the theory is essential, the real understanding comes from working through examples.
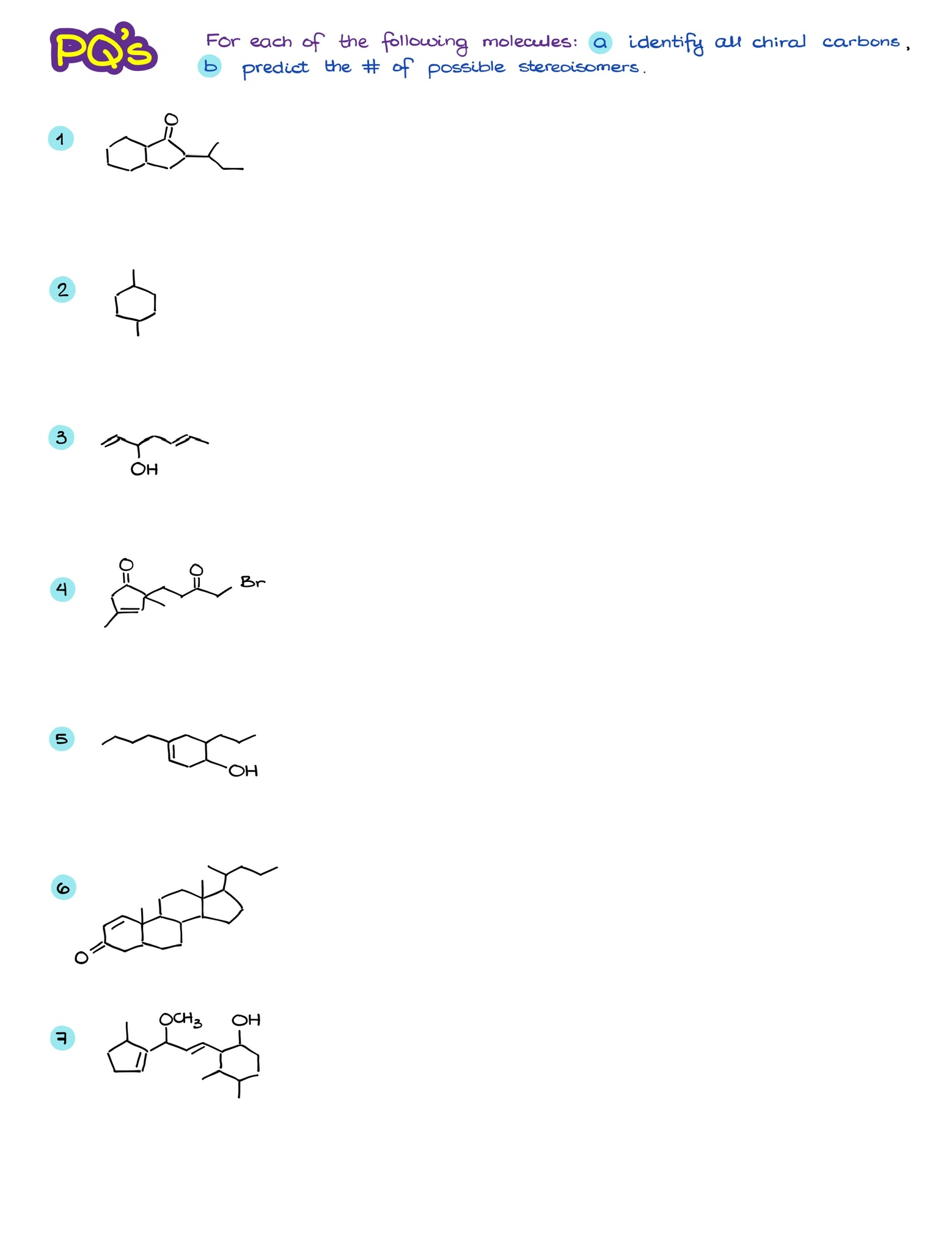
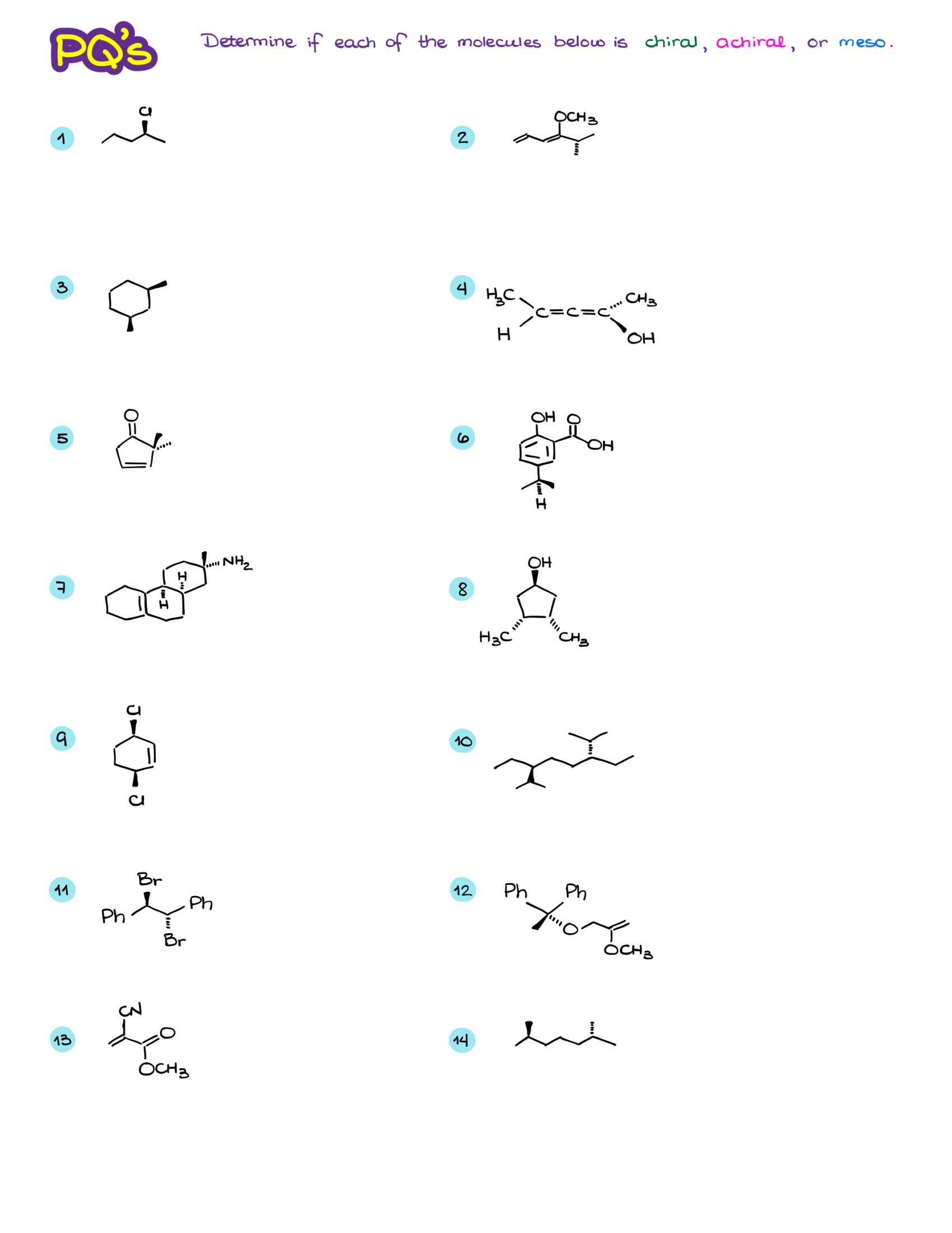

Dear Victor,
I’m doing on my own learning organic chemistery and I’m now busy with this topic: Meso compounds. Could you please publish the answers of the PQ’s online, so that I can check myself? I really would appreciate that.
Thank you in advance for your help.
Yours truly,
Fleur
All practice questions I have on the website have answers posted. Sometimes they are on the same page, sometime they are on a separate page. You’ll need a membership to see them.