Grignard Reagent and Grignard Reaction
Victor Grignard is best known for two things. One hell of a mustache he sports on every picture of him on the Internet, and the reaction named after him which earned him a Nobel Prize in 1912. In this tutorial, we’ll discuss the details of the Grignard reaction, how to approach those on the test, and common mistakes to avoid.
Formation of the Grignard Reagent
Before we jump into the discussion of the Grignard reagent reactivity, let’s talk about how we make the Grignard reagent itself and what exactly it is.
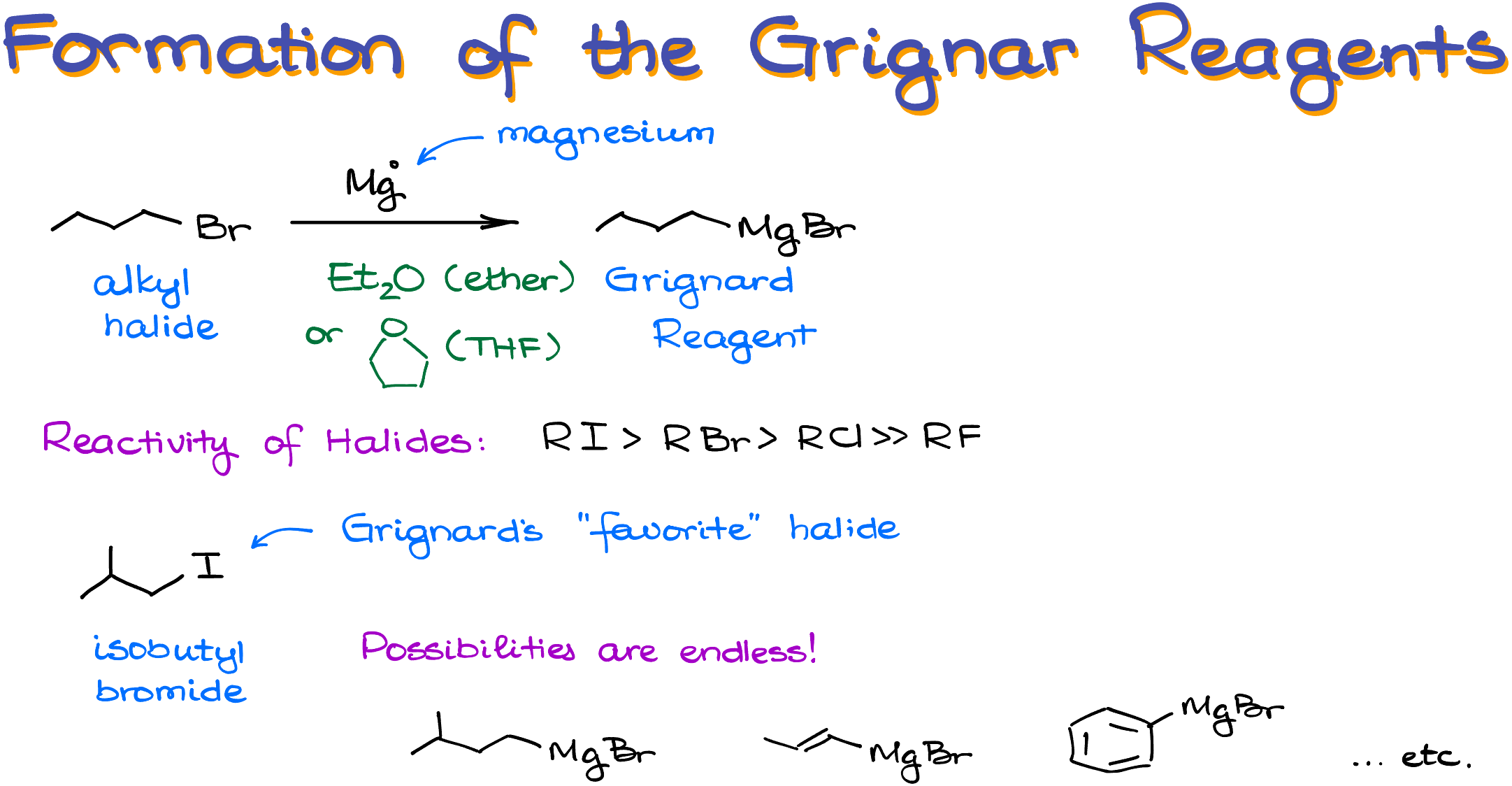
We make the Grignard reagent by reacting an alkyl halide with magnesium shavings in anhydrous ether or THF (tetrahydrofurane). The exact mechanism of this reaction goes beyond the scope of this tutorial, but if you really want to know, let me know in the comments below, and I’ll make a video about this mechanism as well.
From the reactivity perspective, the iodine and bromine containing halides are the most reactive, chlorides are ok but not the best choice for the reagent, and fluorides are comparatively unreactive, so you’ll never see those in your course. In the original research, Victor Grignard used isobutyl iodide.
Another important thing to keep in mind is that we’re virtually not limited by the nature of our alkyl halides. Your halogen can be a simple chain with a halogen on an sp3-hybridized atom, or a complex molecule with the halogen on even an sp2-hybridized atom! The sky is the limit here, making the Grignard reagent truly a Swiss army knife of nucleophiles!
Grignard Reagent Structure
When it comes to the Grignard reagent structure, things are not as simple as they may seem. Good news is, within the scope of a typical sophomore organic chemistry, we don’t need to know the gory details of how the Grignard reagent really looks like. So, we’ll use a simplified approach to its structure which works just fine for our purposes.
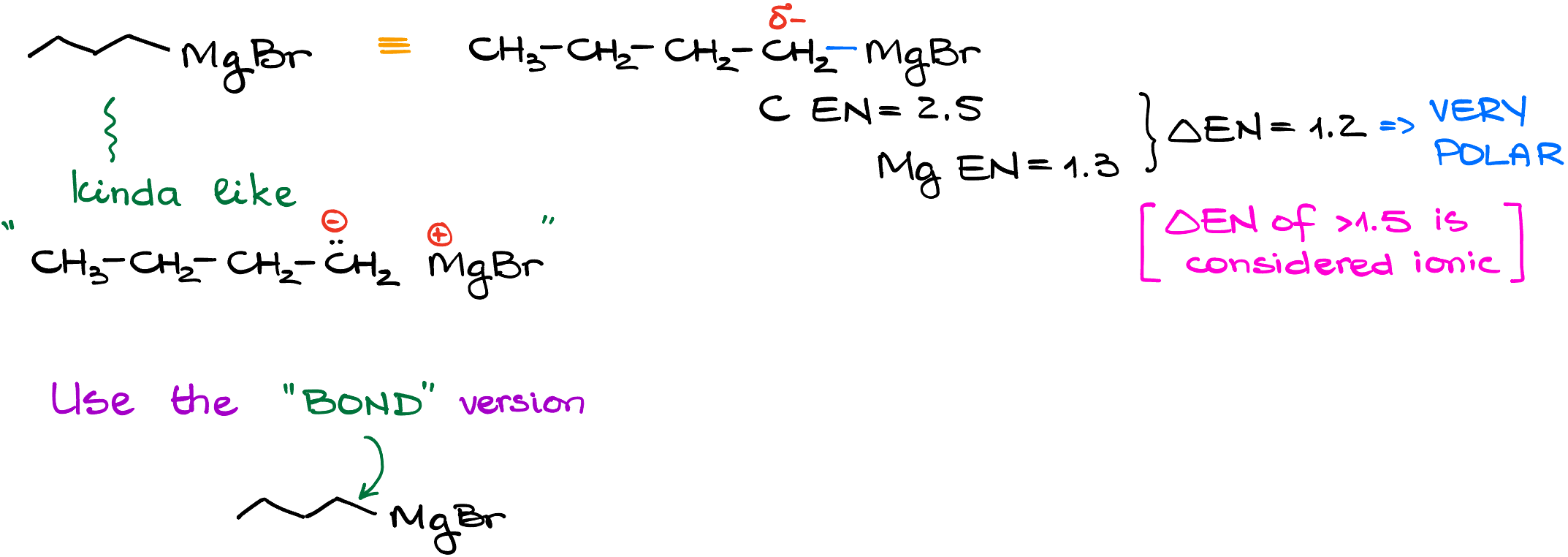
So, due to the direct C-Mg bond, carbon atom has a very high electron density on it. The electronegativity of magnesium is about 1.3, while the carbon’s electronegativity is roughly 2.5. This means that we have a very significant difference in the electronegativity making this bond so polar, it’s close to an ionic bond in its behavior.
So, you can think of a Grignard reagent as, essentially, a carbanion in terms of its reactivity and overall behavior. Depending on how your textbook and your instructor wants to treat it, we can see it depicted as an ionic pair (which is an oversimplification), or as a covalent bond between carbon and magnesium. In this tutorial, I’ll stick to the covalent bond depiction.
Reactivity of the Grignard Reagent
As I’ve mentioned earlier, due to the high electron density around the carbon atom and a carbocationic character of the organic portion of the Grignard reagent, these substances are very basic and very nucleophilic. So, first, let’s look at the acid-base properties of the Grignard reagent.
Acid-Base Properties of the Grignard Reagent
Let’s look at a reaction between the butyl magnesium bromide and a hypothetical acid of some sort.
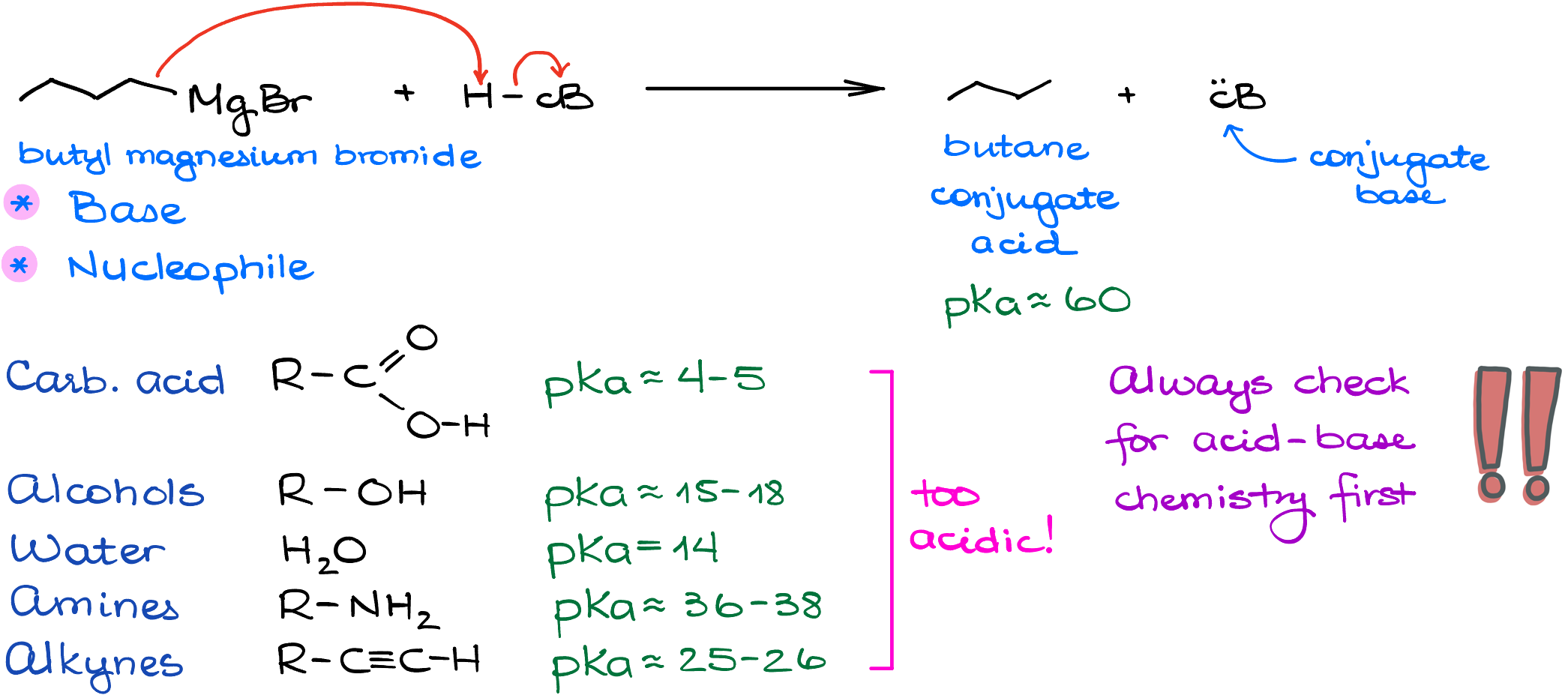
The pKa value of our conjugate acid in this reaction is somewhere in the vicinity of 60. This means that anything with the pKa lower than that will be acidic enough to easily react with our Grignard reagent. If we look at all common acidic (and not so acidic) species we commonly see in organic chemistry: carboxylic acids, alcohols, water, amines, even terminal alkynes—they all are too acidic to peacefully coexist with the Grignard reagent! Which means that when you’re working with the Grignard reagent, you shouldn’t have anything even remotely acidic anywhere in your system.
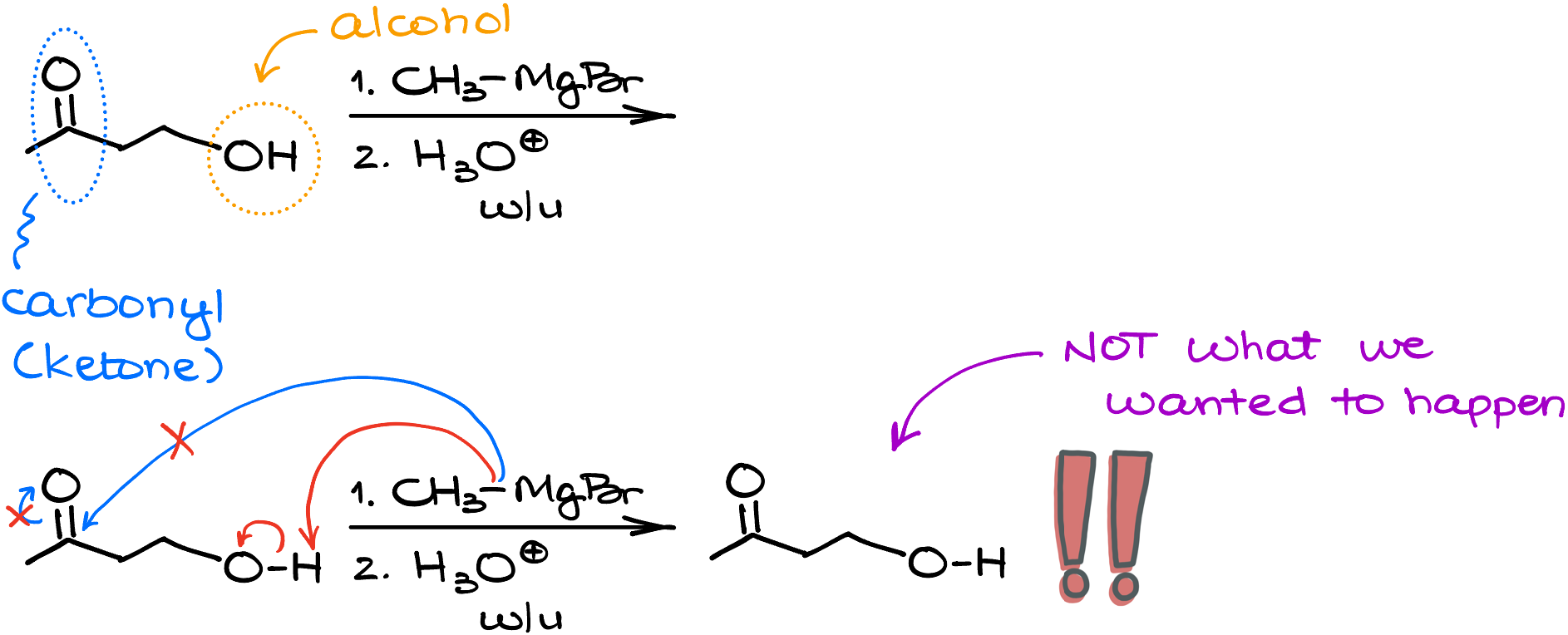
Remember, the proton transfer reactions have a very low activation barrier, so they will typically happen before anything else can happen to your reagents. So, if let’s say, you have a molecule with a carbonyl and an alcohol functional group, the alcohol will react with the Grignard reagent before the carbonyl. This is also why we always must emphasize the anhydrous conditions for the reaction, as water kills the Grignard reagent. This is also a very common trick instructors love to implant into the tests hoping you won’t notice an acidic group and go with the nucleophilic attack instead of the proton transfer.
Now, if the acid-base chemistry is not an issue, we can actually talk about the namesake reaction itself.
The Grignard Reaction
The Grignard reaction truly revolutionized the field of organic chemistry when it was discovered. Victor Grignard discovered it in the 1900 as a part of his doctoral, and it was such a big deal, that he got a Nobel Prize for it in 1912. Just think how cool it would be to get a Nobel prize for your PhD work just 10 years after you get your PhD! Most people barely have a tenure at that point in their career. But I digress.
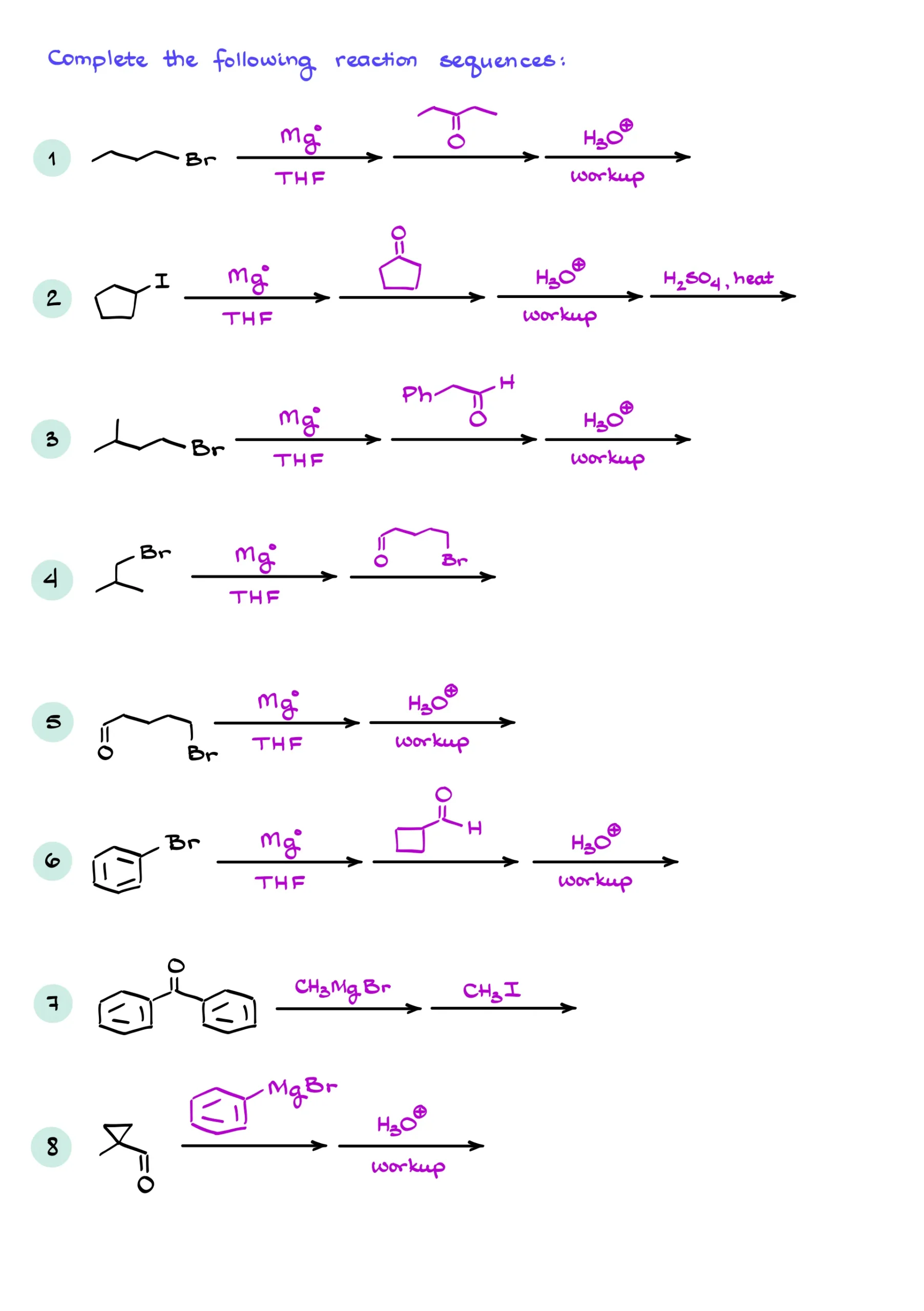
So, what exactly is the Grignard reaction? The classic Grignard reaction is the reaction between the Grignard reagent and a carbonyl like an aldehyde or a ketone giving a corresponding alcohol. Since the reaction is done in highly basic conditions, we’ll initially end up with the alkoxide, which we then protonate in the acidic workup step.
The reaction is extremely useful as it makes a new carbon-carbon bond. So, you can easily construct complex molecules from simple ones. The resulting product is an alcohol, so you can further functionalize your molecule using the alcohol chemistry.
Mechanism of the Grignard Reaction
Let’s look at the mechanism details of this reaction. Since the Grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones.
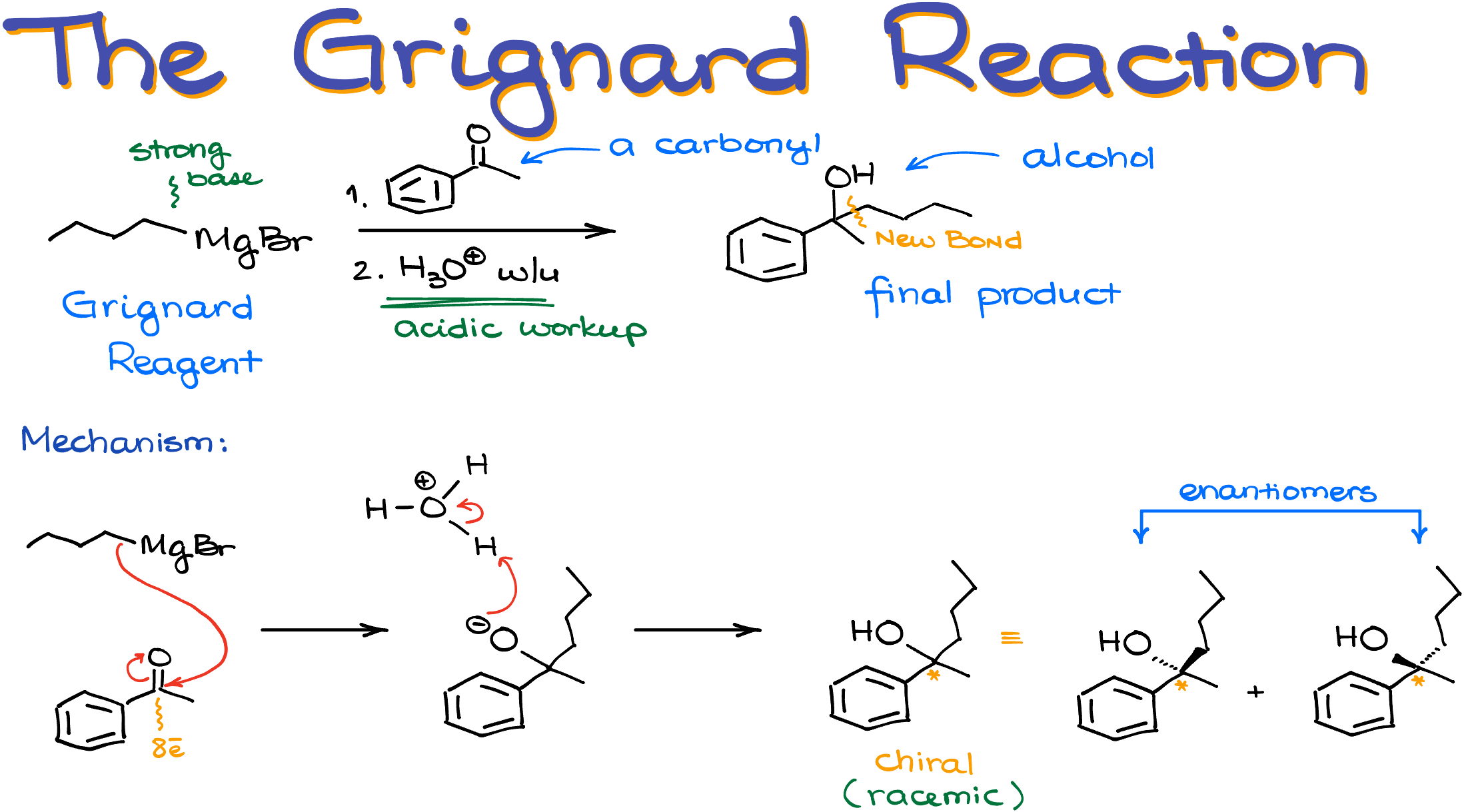
Here, I have a reaction between butyl magnesium bromide and acetophenone. The reaction starts with the nucleophilic attack from the Grignard reagent onto the carbonyl. Traditionally, we show it by taking the electrons from the C-Mg bond and pushing them towards the carbon of the carbonyl. However, if your instructor likes to show it as a carbocation, we’ll then show an electron pair on the carbon atom instead of the covalent bond between carbon and magnesium. As I mentioned above, I’ll stick to the “bond” version of the mechanism.
Here’s also the important thing to keep in mind—since carbon of the carbonyl is already fully satisfied with the electrons and has a full octet, we cannot just add those electrons to it and make a new bond. If we want that carbon to accept those electrons, it needs to push some electrons away. And that’s precisely what it does with the π-bond to the oxygen. Remember to do that when you’re working through the mechanism of this reaction, or you’ll end up with a carbon with too many bonds around it.
This step makes a new carbon-carbon bond and a negatively charged oxygen.
In the next step, we’re going to do the acidic workup protonating the O-. The acidic workup is always going to be the second step in this reaction. Some instructors skip it or “assume” it but it’s always there even if they don’t show it.
In this case, the resulting alcohol is chiral. At this point in your course, your instructor will most likely expect you to indicate the stereochemistry of your products when relevant. So, make sure you do so and don’t forget about it. Here, if I wanted to indicate my stereochemistry, I would either write “racemic” under my molecule or show both stereoisomers which are enantiomers for this molecule.
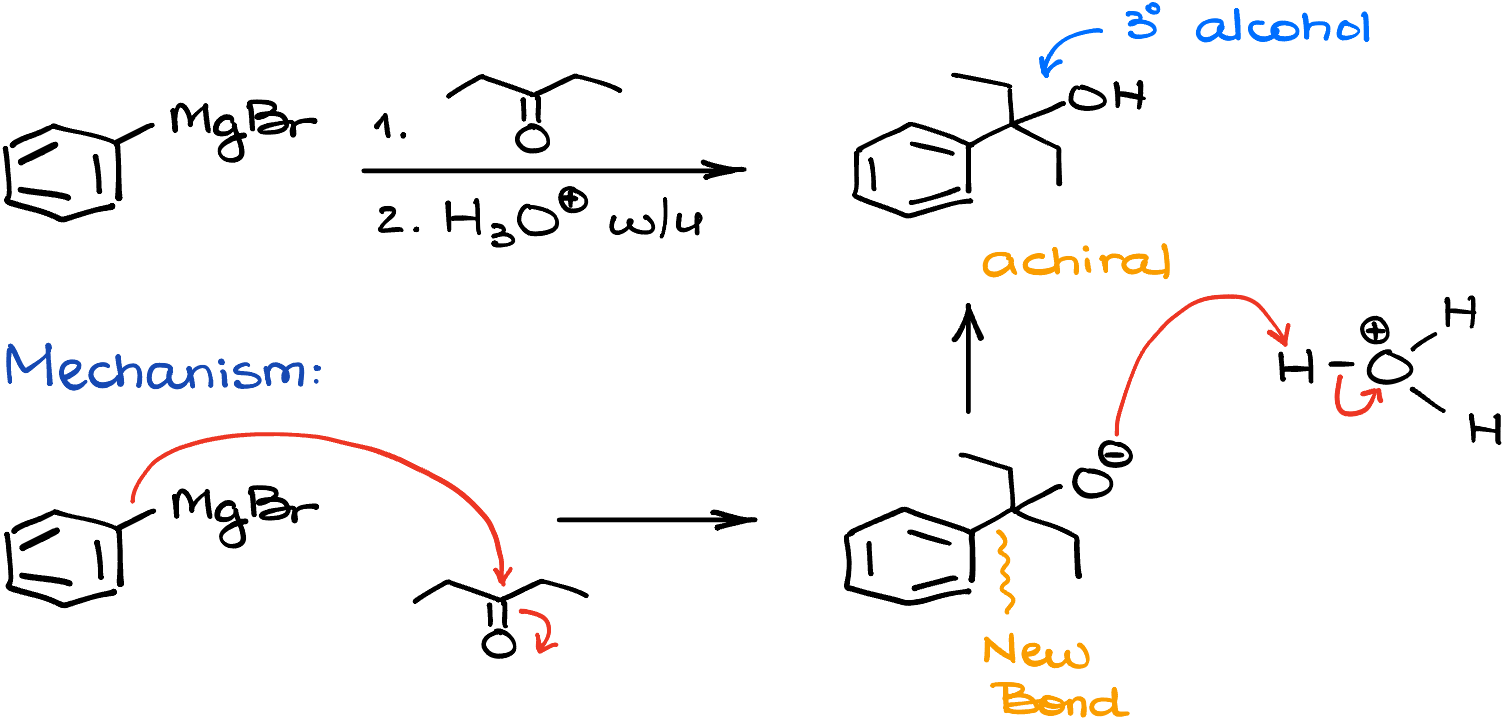
Let’s look at another example. In this case we’re reacting the phenyl magnesium bromide with pentane-3-one. This reaction makes a tertiary (3°) alcohol similar to the last example. However, in this case, we’re going to get an achiral molecule. And the mechanism is going to be very similar to the previous example as well. We’ll start by attacking our carbonyl making a new carbon-carbon bond. And once that part is done, we’ll protonate our molecule in the acidic workup step.
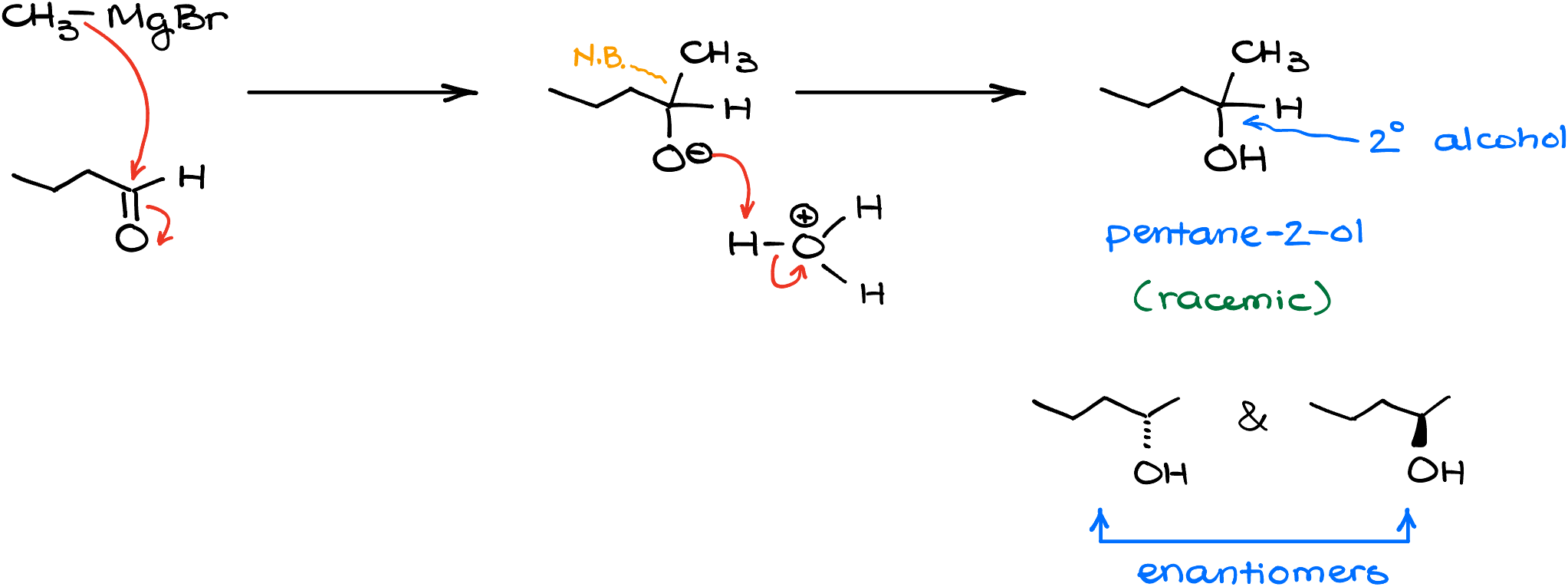
How would this reaction proceed with an aldehyde? Well, a reaction with an aldehyde gives us a secondary (2°) alcohol. For instance, in the reaction of the methyl magnesium bromide with butanal, we’ll get a pentane-2-ol after the acidic workup. This molecule is chiral as well, so I’ll say “racemic” or show both possible stereoisomers.
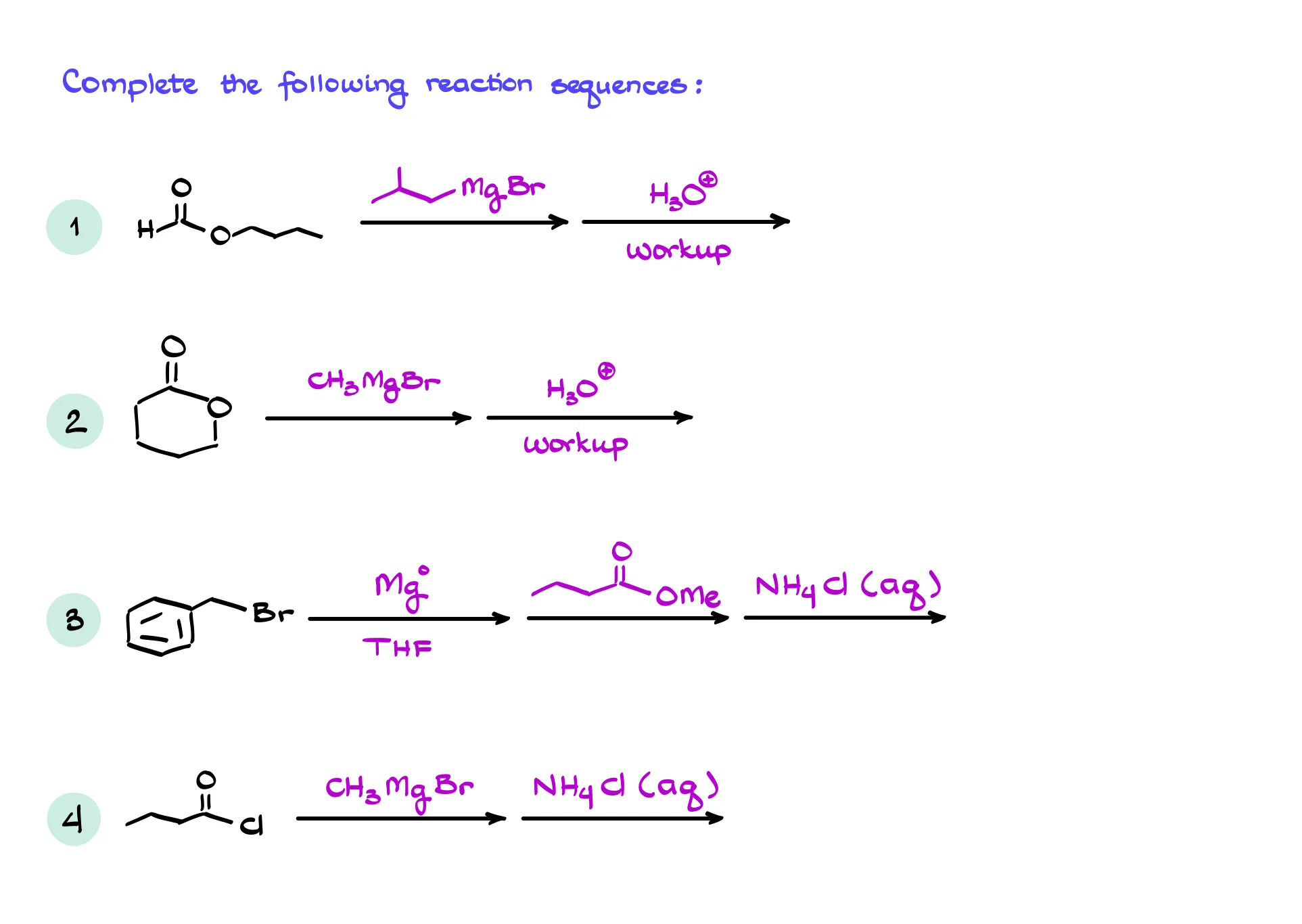
Reaction of the Grignard Reagent with Esters and Acid Chlorides
The Grignard reagent can react with a whole range of various electrophiles. Another common example is going to be the reaction with esters and acid chlorides.
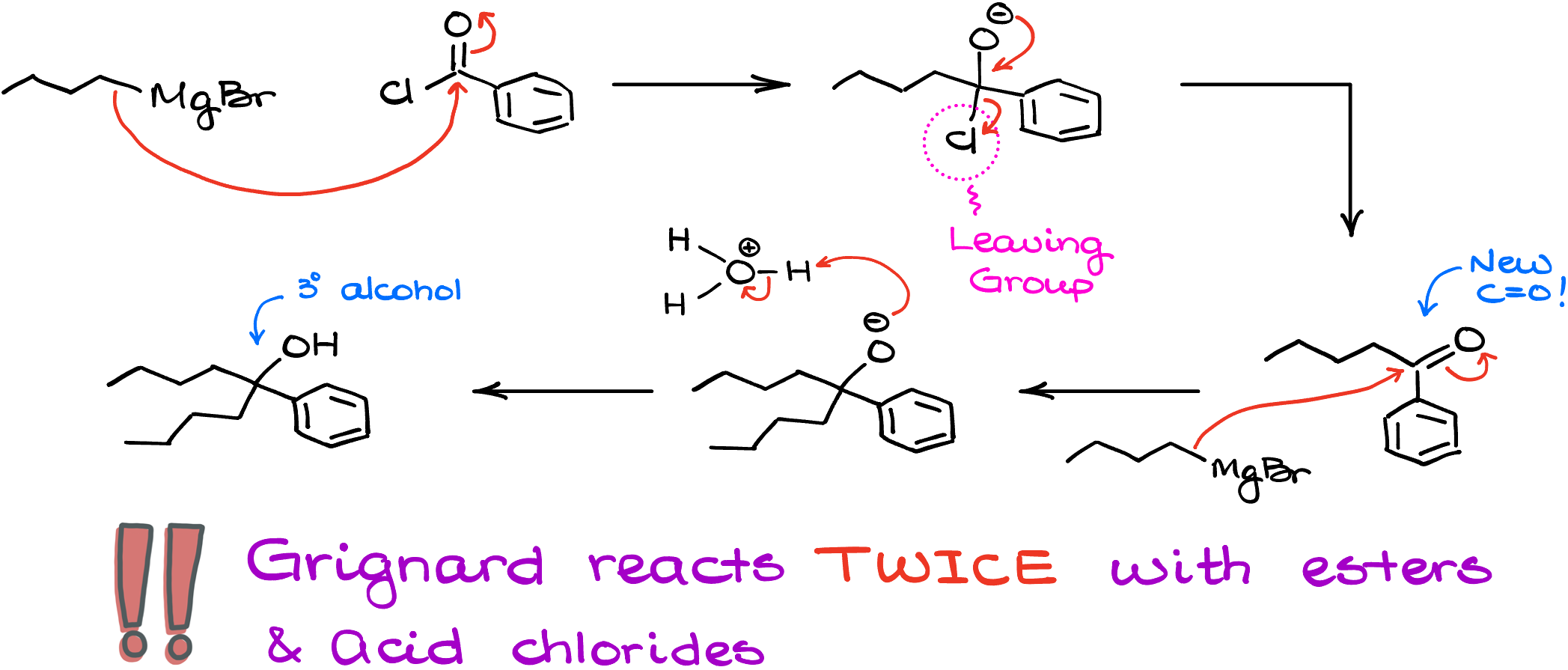
If I take a Grignard reagent, butyl magnesium bromide, and react it with, for instance, benzoyl chloride, the first step in this reaction is going to be exactly what we would expect from it—the nucleophilic attack on the carbon of the carbonyl. However, we now have a leaving group which can be easily pushed off our molecule. This recreates the C=O and the reaction can happen one more time! So, the next equivalent of butyl magnesium bromide will come in and attack again. This makes another carbon-carbon bond yielding the alkoxide like if we did this reaction with a ketone. And only after that we’d be able to do our acidic workup giving us the tertiary (3°) alcohol as the final product. So, important thing to remember here is that the Grignard reaction with acid chloride happens twice. And for as long as you’re working with a simple Grignard reagent, you won’t be able to stop if after the first round of addition. There are, of course, ways how we can prevent it, but it would require special conditions and additional components in our reaction mixture which goes beyond the scope of this tutorial.
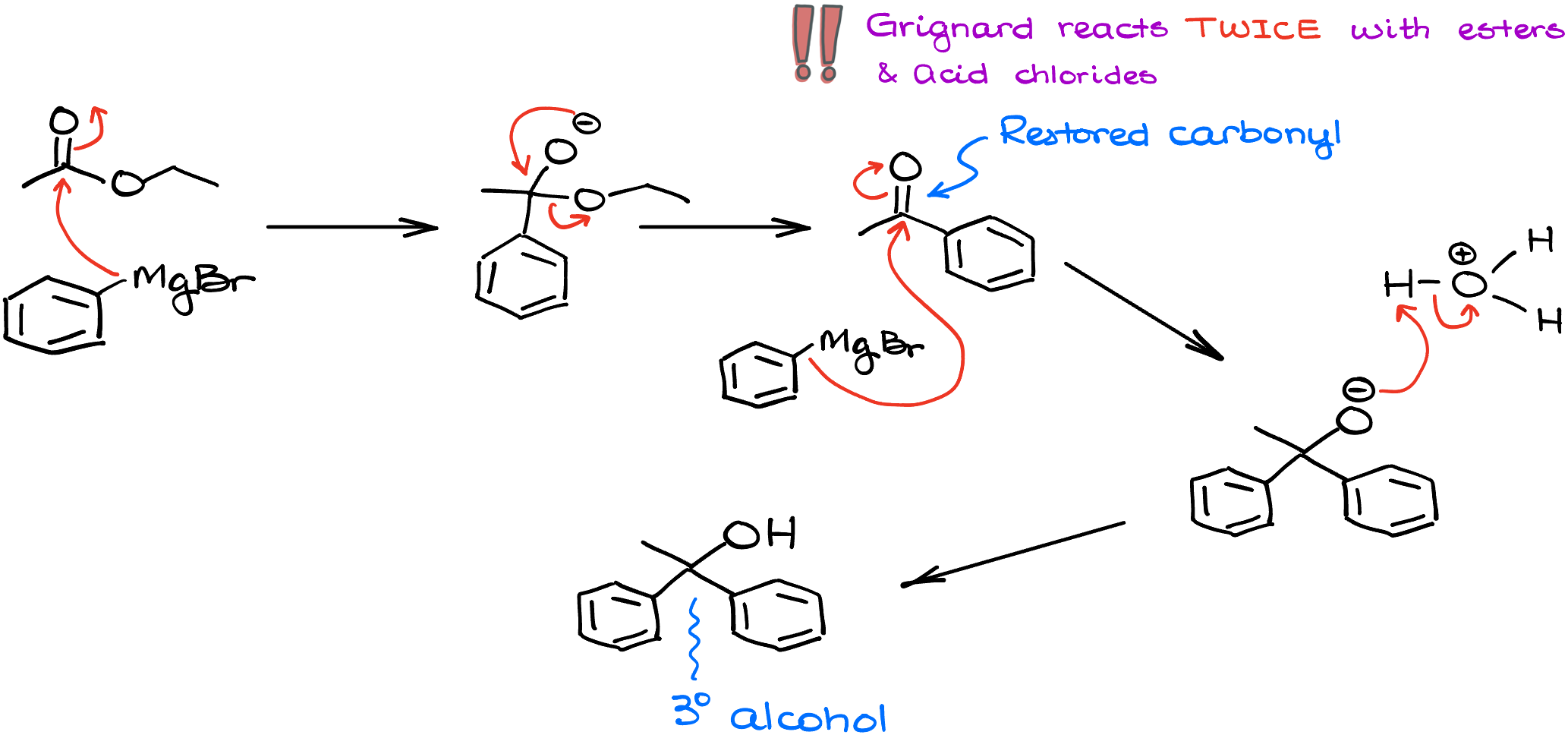
What about the esters? The reaction proceeds in a similar fashion. If I treat ethyl acetate with phenyl magnesium bromide, I’ll first do the first round of the addition giving me the negatively charged intermediate. That intermediate will push away an alkoxide leaving group restoring the C=O and the reaction can happen one more time with another equivalent of phenyl magnesium bromide, eventually giving us a tertiary alcohol as a final product. So, the idea here is the same—reaction happens twice and you end up with a tertiary alcohol.
While there are other variations of this reaction with carboxylic acid derivatives, I’ll limit our discussion here just to these two reactions. But you’ll also learn how the Grignard reagent reacts with nitriles and amides later in your course.
Reaction of the Grignard Reagent with Epoxides
Another variation of the Grignard reaction you are likely to encounter in your course, is the reaction with an epoxide. This is a useful reaction from the synthetic perspective as it can give us a new carbon-carbon bond one carbon away from the -OH group in the resulting alcohol.

So, if I react phenyl magnesium bromide with 2,2-dimethyloxirane, I’m going to attack the less substituted carbon of my epoxide. This creates a new carbon-carbon bond opening the epoxide ring. And since the oxygen is still connected to the other atom, it makes an alkoxide with the oxygen one atom removed from the place where we have attached our Grignard reagent. Of course, here like in all of these reactions, we’ll need to do an acidic workup to protonate the resulting anionic species.
Concluding Thoughts

The Grignard reaction is an incredibly versatile method of making new carbon-carbon bonds. For many years, it was the premiere method of the carbon-carbon bond formation. It’s made such a profound impact on the field of organic chemistry, that early 1900’s to about 1960 is known as the Grignard era of organic chemistry. And while this reaction is over 120 years old, it is still regularly used in the modern research and synthetic strategies in organic chemistry. It’s also going to be one of the premier carbon-carbon bond-making methods in your course. So, whenever you’re looking at a synthesis problem and see that there was a new carbon-carbon bond added, chances are, the key step in that synthesis is the Grignard reaction.
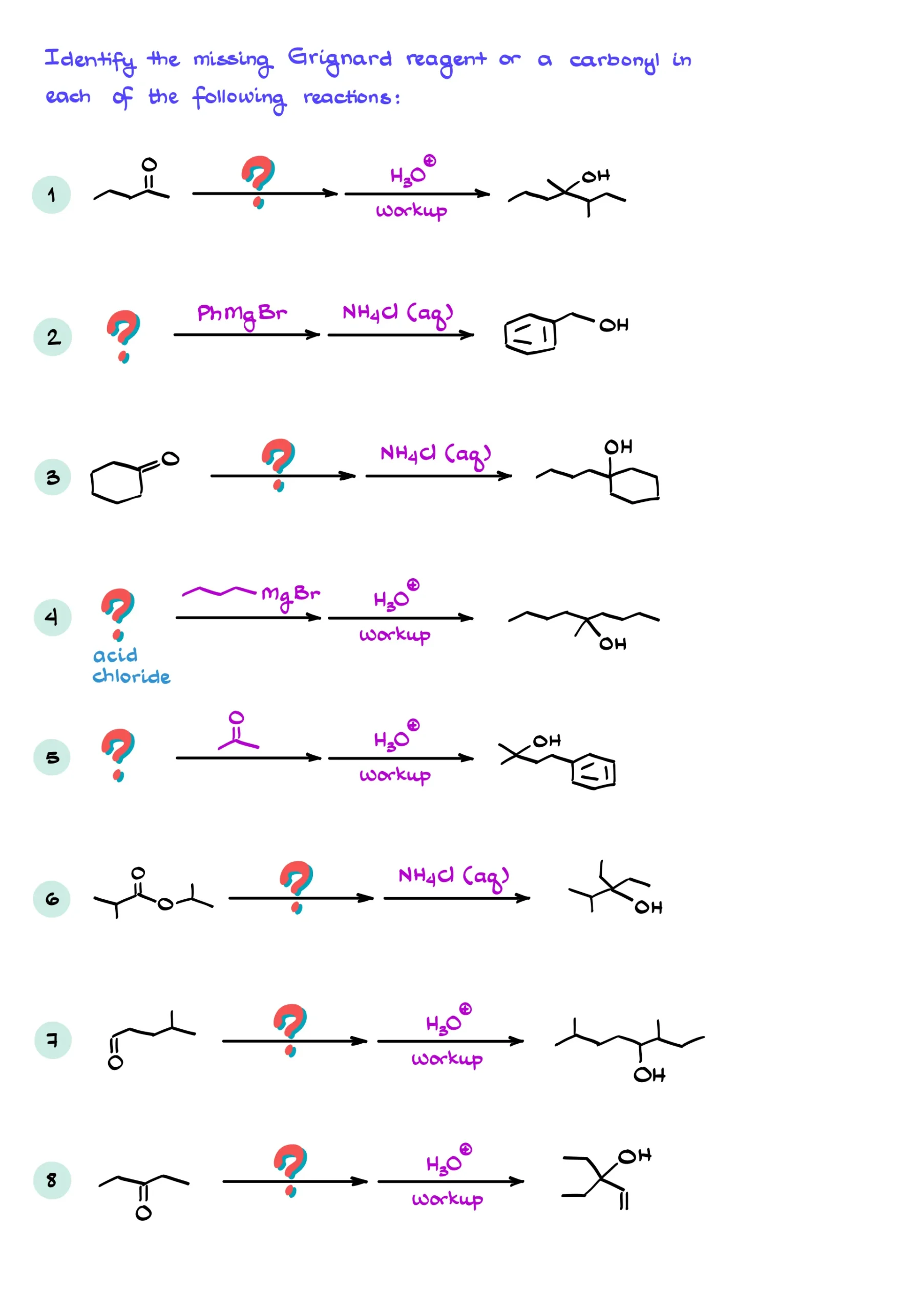
Practice Questions
Answers
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!