Grignard Reaction of Nitriles
Reaction of Grignard reagents with nitriles is, probably, one of the most overlooked reactions in the sophomore organic chemistry curriculum. However, this reaction is one of the few methods that you can use to make a ketone using the Grignard reaction. In this tutorial we’ll look at the mechanism of the Grignard reaction of nitriles and how to use this reaction in synthesis.
General Scheme of the Reaction of Nitriles with Grignard Reagents
As the Grignard reagent is a powerful nucleophile, it can react even with the weak electrophiles like nitriles. However, since the Grignard reagents cannot attack negatively charged molecules, the reaction unlike in the case with many other carboxylic acid derivatives only occurs once.
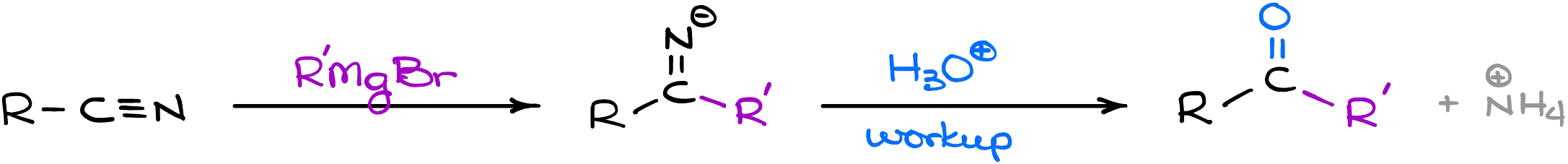
So, while we normally think about the Grignard reaction as a method of the alcohol synthesis, this variation makes ketones.
Mechanism of the Grignard Reaction with Nitriles
Mechanistically, this reaction begins like any other Grignard reaction by the nucleophilic attack on the nitrile. The result of this attack is the formation of the negatively charged iminium intermediate. And since the Grignard reagents cannot react with negatively charged species, we’re not going to see the second attack.
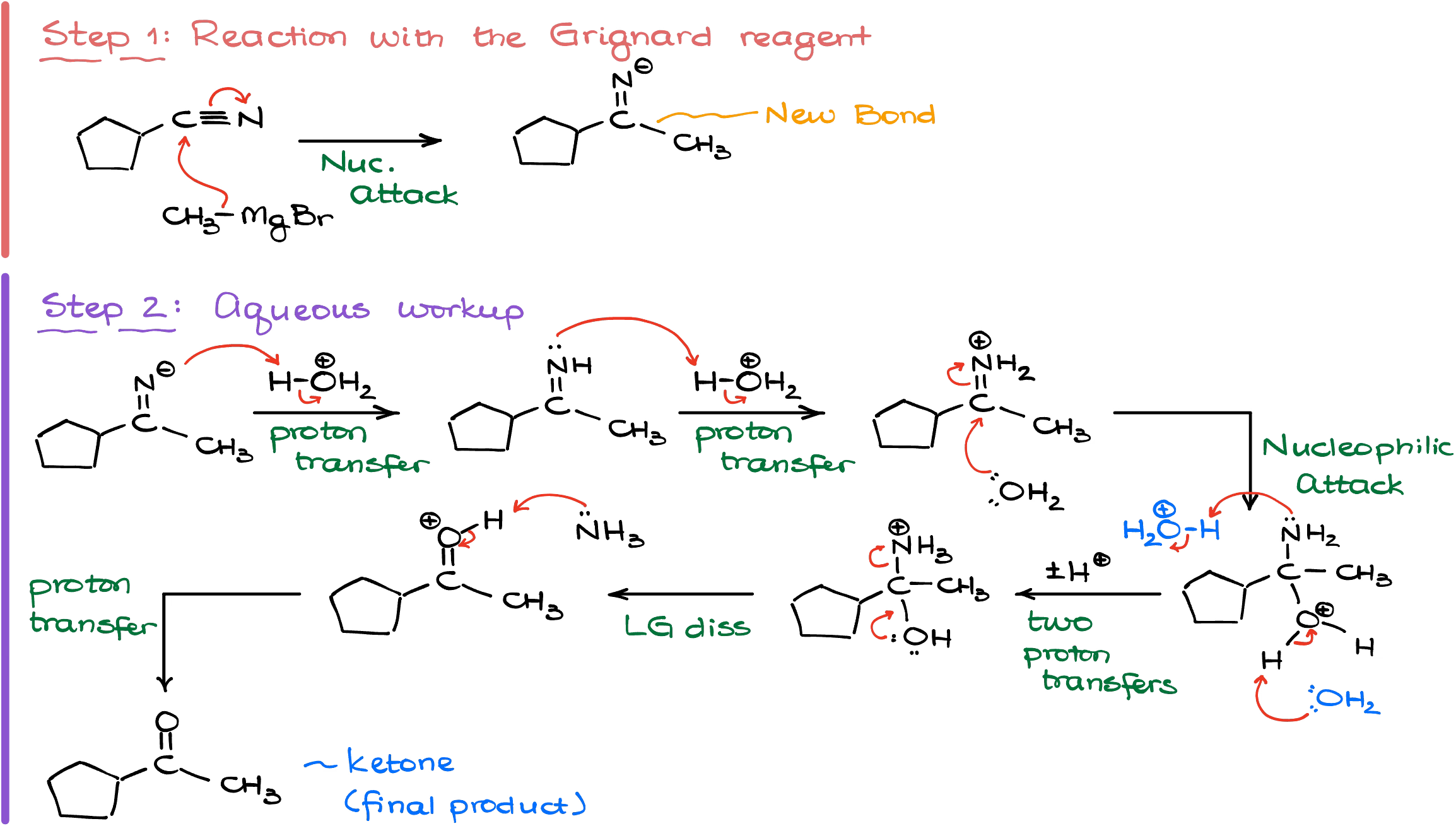
The aqueous workup in this case gives us an imine. But since imines hydrolyze extremely easy, we typically don’t get the imine as the final product but rather we get the product of the imine hydrolysis—the ketone.
Examples
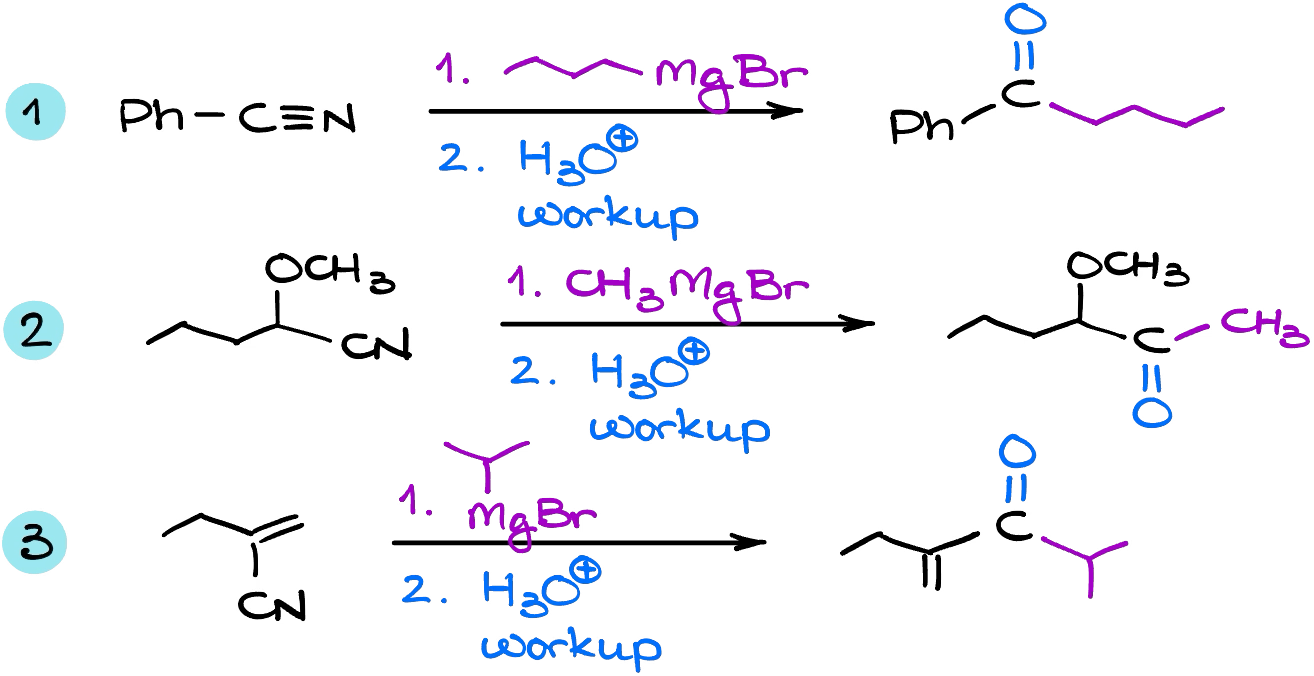

if we took excess of grignard reagent then will the ketone further react to form a tertiary alcohol?
No, it’s not the matter of the excess of Grignard’s but rather the Grignard reagents are just not nucleophilic enough to react with a negatively-charged intermediate we get after the first attack. So, even if you use excess of the Grignard reagent, you’re still not going to be able to effectively push the reaction past that point.