Formation of Hydrates from Aldehydes and Ketones
Since aldehydes and ketones are quite electrophilic, they can react with a vast number of nucleophiles. A common reaction of aldehydes and ketones is the reaction with water giving corresponding hydrates. And while technically this type of a functional group is a geminal diol, traditionally, we call them hydrates because you’re always going to be making them via a reaction of carbonyls with water.
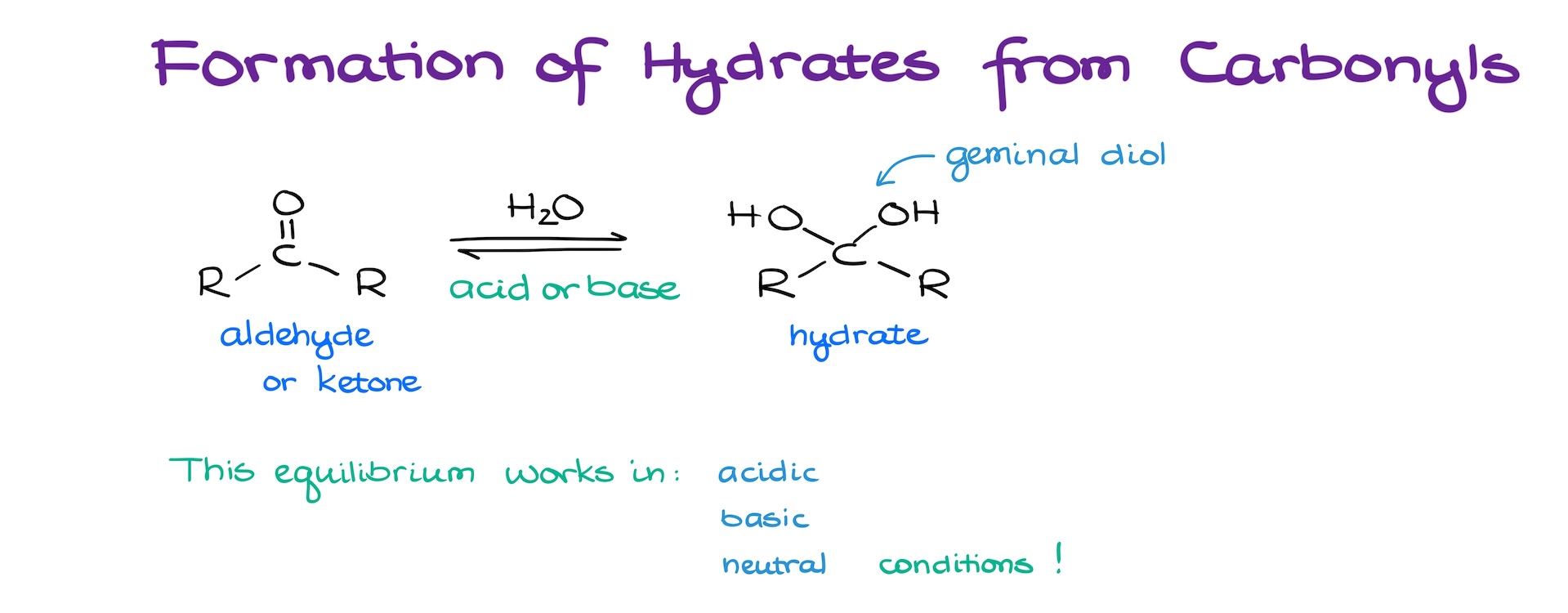
The tricky part of this equilibrium is that it works in neutral, acidic, and basic conditions. So, let’s start by looking at this equilibrium in neutral conditions first.
Neutral Conditions
As I’ve mentioned before, carbonyls are electrophilic. So, if I took the acetone molecule and reacted it with water, we would expect a nucleophilic attack from the water molecule on our carbonyl giving us the zwitterionic intermediate.
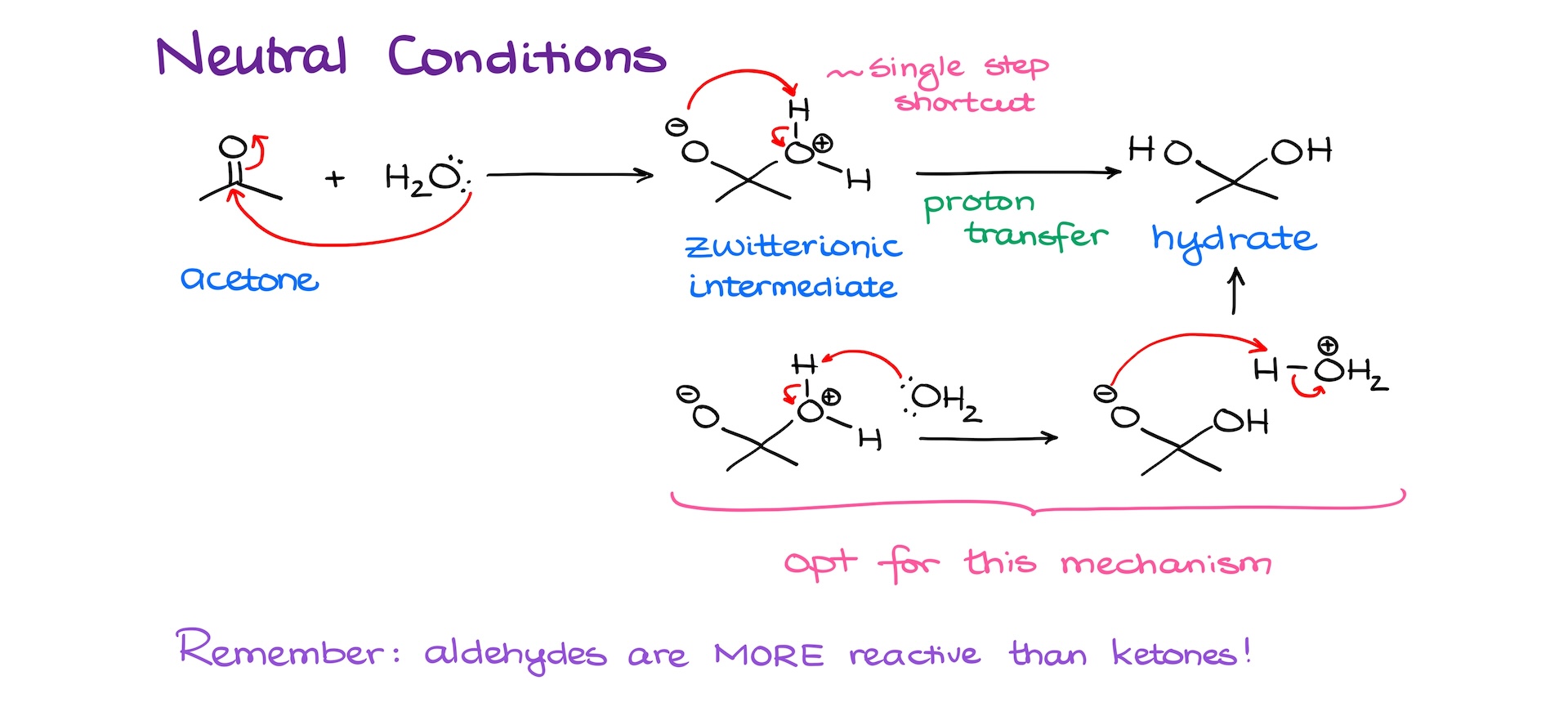
Next, we’ll have a proton transfer removing an extra proton from one of our oxygens, and adding a proton onto the other oxygen. Some textbooks and instructors show this as a single step where the proton just hops from one oxygen to another one. Strictly speaking, while this is a common shortcut, it’s not 100% correct as it violates the orbital symmetry rules that essentially prohibit the 4-member transition state that this interaction would require to go through.
A more correct way to show this proton transfer would be by using a water molecule as a chaperone. In this case, a water molecule would first deprotonate the oxonium ion, and then carry the extra proton to the negatively charged oxygen neutralizing it and yielding the hydrate as the final product. However, if your instructor uses the shortcut like what I showed above, I suppose, you can use it too. Just remember, it’s not a “universally accepted” shortcut and you may lose points if you do it outside of your class.
Acidic and Basic Conditions
Now, on average, aldehydes are around 1000 times more electrophilic than ketones give or take. This of course depends on the structure of our aldehydes and ketones. But even though the carbonyls might be electrophilic, water is not a very good nucleophile. So, this reaction is typically catalyzed by either acids or bases.
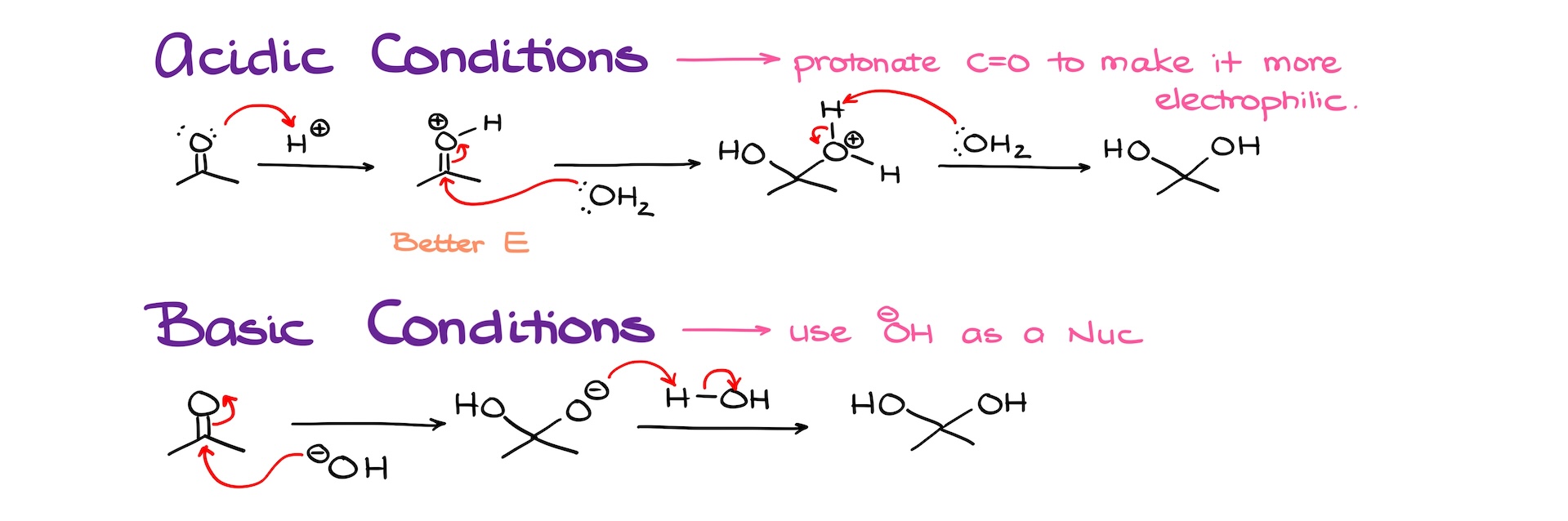
In acidic conditions, we can protonate our carbonyl, taking it from a good electrophile, to an amazing electrophile because of the positive charge. Now, it’s going to be more inclined to react with nucleophiles even if it is a poor nucleophile like water. In this case, the nucleophilic attack going to happen much easier, yielding the hydrate after the proton transfer to neutralize the intermediate.
In basic conditions, we’ll use the hydroxide anion as a nucleophile, which is a much better nucleophile than water due to the negative charge that we have on our oxygen. The nucleophilic attack in this case produces a negatively charged intermediate, that we are going to neutralize by grabbing a proton from water regenerating our base catalyst and yielding the hydrate as our final product.
So, in the end, it doesn’t matter which conditions we use, we always going to get the same product. Using either acid or base as a catalyst going to make the process faster. But frankly speaking, this reaction is so fast as is, that arguing the utility of the catalyst here is kinda pointless.
Equilibrium State and Hydrate Stability
What’s not pointless though is the fact that this reaction is an equilibrium. And the state of this equilibrium depends on the structure of the original carbonyl we use. Normally, when we look at any equilibrium, we know that we can easily influence it using the Le Chatelier’s Principle. And yes, you can also do it here to some extent. But the structure of the carbonyl compound would still influence the outcome the most.
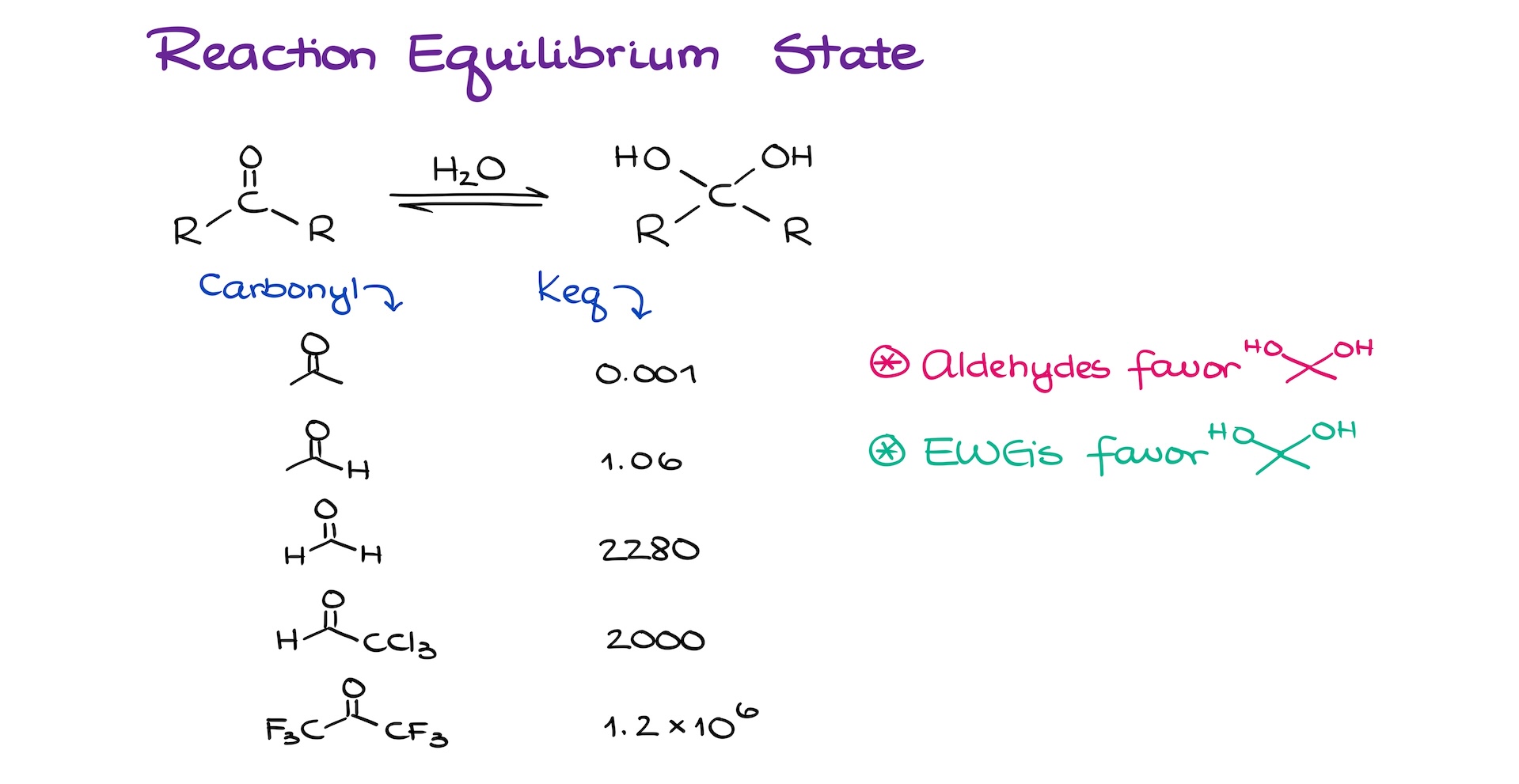
Let me illustrate it with an example of some equilibrium constants. Here, I have a generic equilibrium representing some sort of a carbonyl, either aldehyde or a ketone, depending on the R’s, reacting with water giving us the corresponding hydrate.
If we look at the equilibrium constants that we’ll get from different aldehydes and ketones, we can see quite a drastic spread of the Keq depending on the structure. Of course, we do not expect you to know or memorize these numbers. However, there are two take-home messages I want you to remember.
First, aldehydes typically favor the formation of a hydrate over the carbonyl from in this equilibrium. And second, Electron-Withdrawing Groups (EWG’s) push equilibrium towards the hydrate as well.
Is it ever going to be relevant in synthesis? Not really. But it is going to be relevant in biological systems and some biochemically relevant reactions you’ll learn in either the very end of organic chemistry sequence or in your biochemistry class. Understanding the formation and decomposition of hydrates is the first stepping stone in understanding the equilibrium of the acetals and hemiacetals which are important for both synthesis and biochemistry.
Decomposition of Hydrates
And talking of equilibrium, I’ve mentioned that the formation of these hydrates is an equilibrium process. This means that we can take the hydrate and break it back into the corresponding carbonyl and water. And of course, we can do it in acidic, basic, or neutral conditions. I’ll quickly go over this mechanism in acidic and basic conditions as those are the two conditions you’re most likely to see on the test.
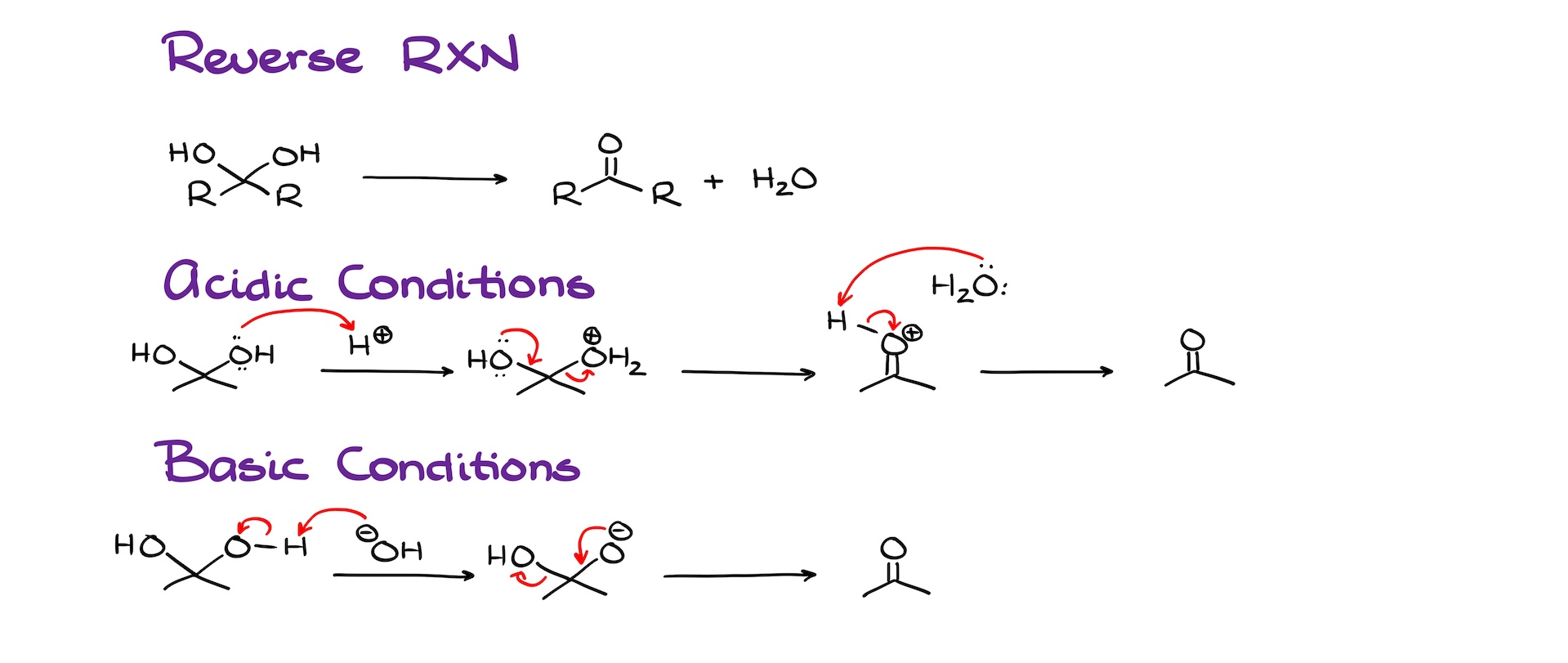
In acidic conditions, we’ll start by protonating one of the -OH groups, forming H2O, which is a good leaving group. Then, water dissociates, and we get a protonated carbonyl intermediate, which quickly loses the proton to a water molecule, and we get our carbonyl.
In basic conditions, we’ll deprotonate our hydrate first, then push away the -OH group making the carbonyl. And you might wanna protest here telling me that -OH is a horrible leaving group. And you would have been correct if we were working in acidic or neutral conditions. Here, however, we’re working in basic conditions. And in basic conditions, -OH can be a leaving group without any problems.
