Formal Charges
Formal charges in organic chemistry is, perhaps, one of the most fundamental bookkeeping devices which is often misunderstood or neglected by students.
Why Formal Charges are Important in Organic Chemistry?
Knowing formal charges can help us understand the reactivity patterns in reactions, find reactive centers, and make sense out of electron flow in the mechanisms. For instance, negatively charged species tend to be the sources of electron density in reactions, while the positively charged species—accept those electrons.
If you’ve started your journey in organic chemistry, you’re well aware that molecules are often depicted with neutral atoms. However, not all atoms remain neutral.
Basics of Bonding Patterns and Neutral Atoms
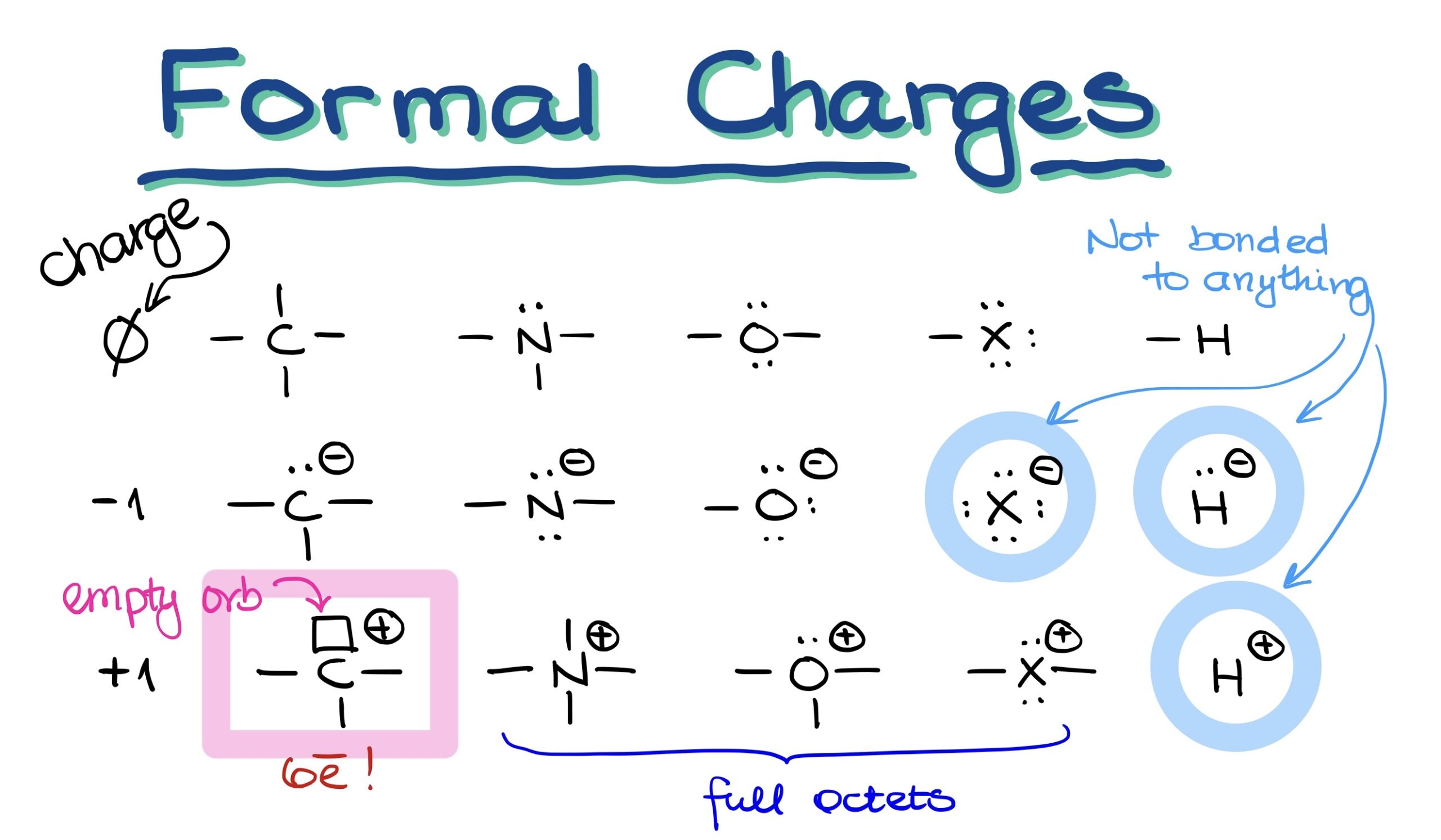
When we think about organic molecules, the first step is to visualize their bonding patterns. For instance:
- Carbon: Always seen with 4 bonds.
- Nitrogen: Exhibits 3 bonds alongside an electron pair.
- Oxygen: Bears 2 bonds and 2 electron pairs.
- Halogens: Usually come with 1 bond and 3 electron pairs.
- Hydrogen: As simple as it gets, hydrogen has just 1 bond.
In these classic examples, the charge on each of these elements is zero.
What Happens When Atoms Are Negatively Charged?
Now, things get a bit more intriguing when atoms have a negative charge. In this scenario, one bond morphs into an electron pair.
- For carbon, this means one bond will change into an electron pair.
- Nitrogen will have 2 bonds and 2 electron pairs.
- Oxygen gets 1 bond but 3 electron pairs.
- Halogens become free-floating ions with 4 electron pairs and no bonds.
- Lastly, hydrogen transforms into a hydride anion.
Notably, negatively charged halide and hydride anions lack any bonds. Thus, they exist solely as free ions.
What About the Positive Charges?
Positive charges add an extra layer of complexity:
- Carbon will have 3 bonds and an intriguing empty orbital (which can be visualized as a small box).
- Nitrogen, a bit unexpectedly, will have 4 bonds.
- Oxygen maintains 3 bonds but keeps one of its electron pairs.
- Positively charged halogens will possess 2 bonds and 2 electron pairs.
- Hydrogen becomes a lone proton.
Here’s an important fact: All heteroatoms (like nitrogen, oxygen, and halogens) will always have a full octet. Even though it’s theoretically possible for heteroatoms to be open-shelled with just 6 electrons, such scenarios are too unstable in organic molecules. The only exception? Carbon with a positive charge. These are termed as ‘carbocations’. Despite their existence, they’re quite unstable.
Highlighting the Significance of Charges
Drawing bond line structures in chemistry can sometimes allow us to implicitly display hydrogens and electron pairs. But charges? They’re never implicit. A common oversight many students make is overlooking these charges. Thus, always double-check your drawings!
To calculate an atom’s charge, you can use the following formulas:
- Official way: Charge = Valence electrons – (½ × Bonding electrons) – Non-bonding electrons.
- Simplified: Charge = Valence electrons – Lines (bonds) – Dots (lone electrons).
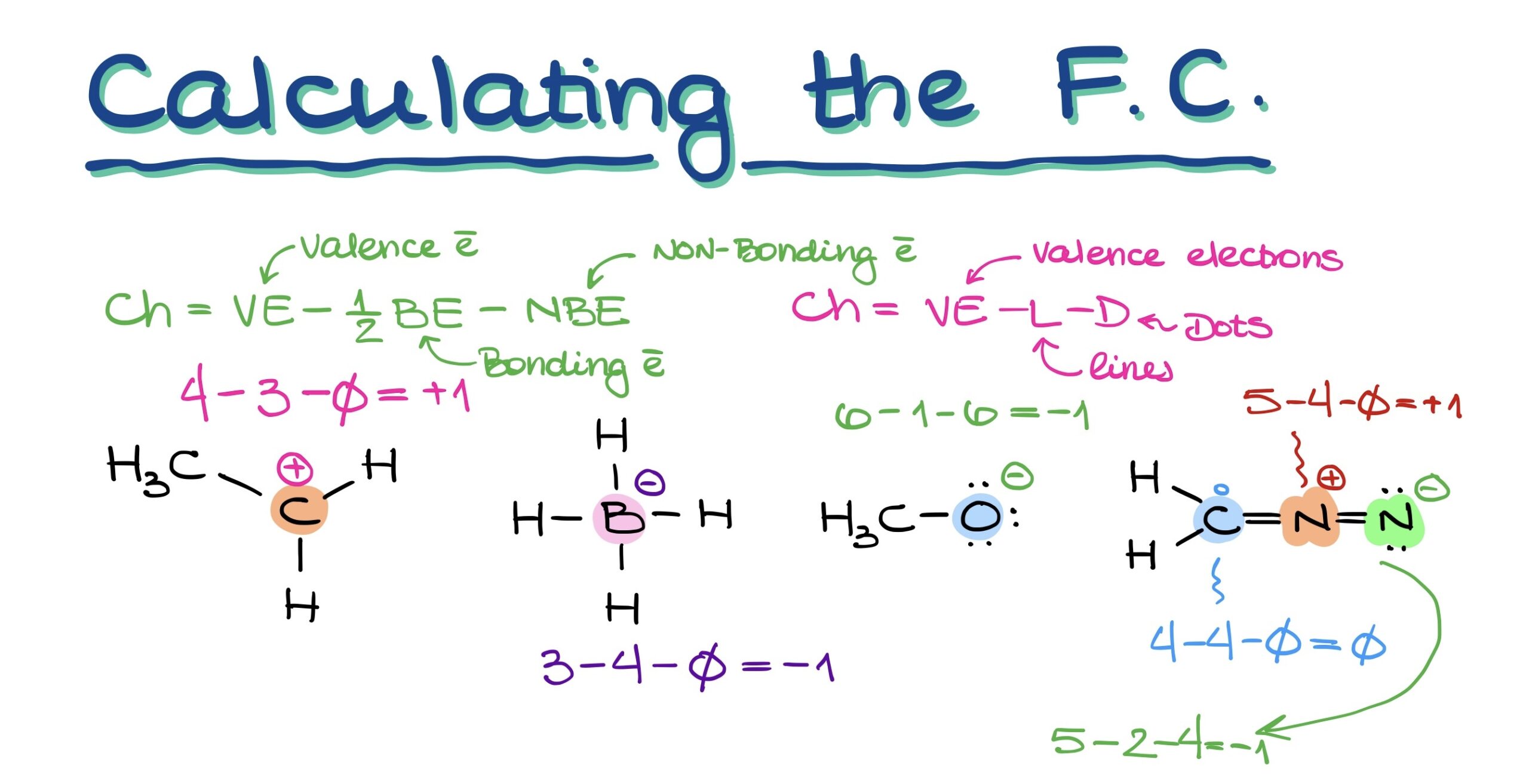
This latter method, with its clear emphasis on subtracting lines and dots from valence electrons, can help you determine the charge on any atom in your molecule effortlessly.
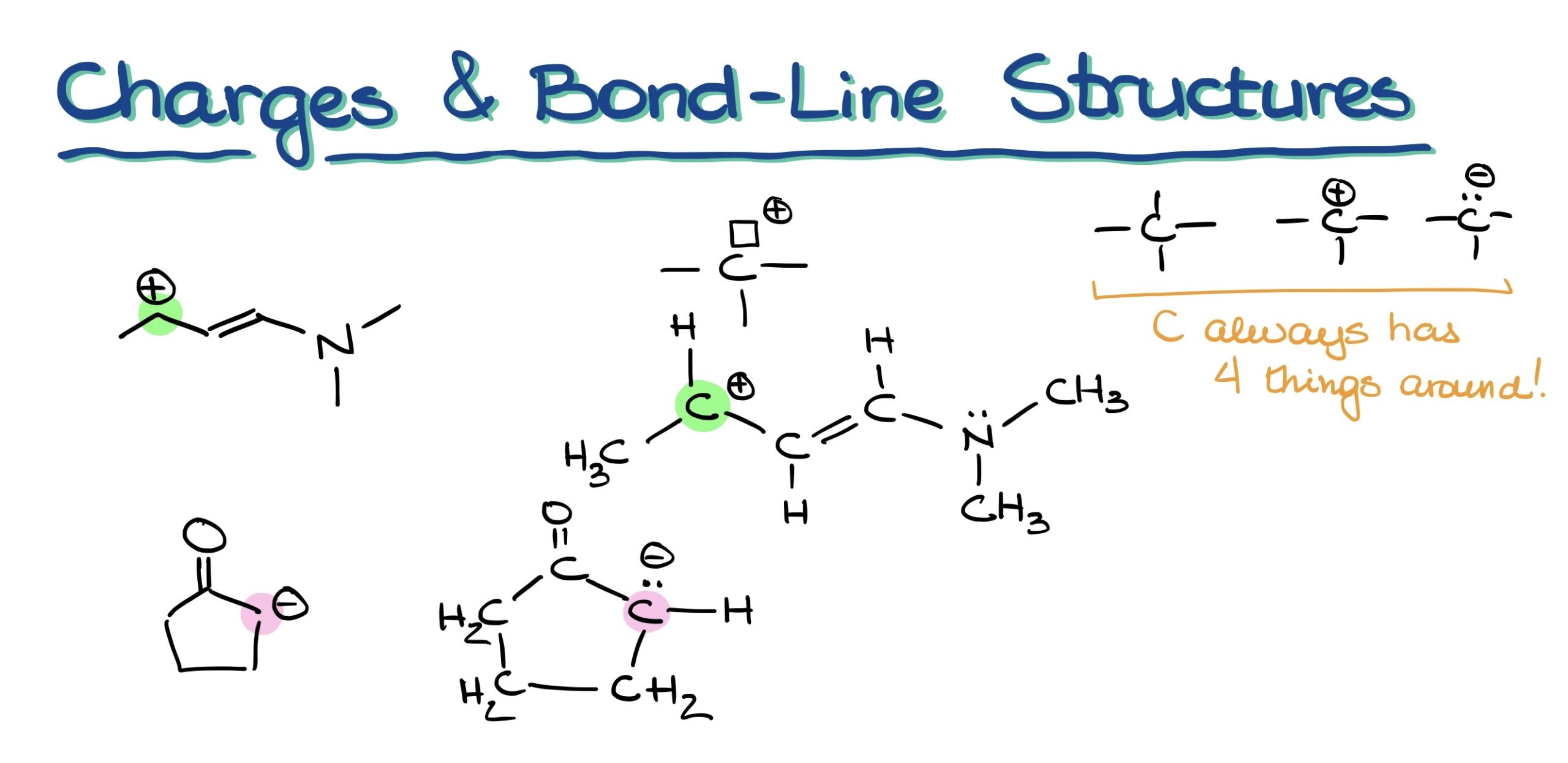
Beware of Large Charges!
A word of caution: While organic molecules can technically bear charges greater than +1 or -1, encountering such is a rarity. If you find an atom with a charge like +3 or -4, double-check your work. Having multiple charges spread across different atoms is feasible, but a single large charge on an individual atom is uncommon.
The Difference Between the Formal and Actual Charge
Now, I’ve mentioned earlier that there’s a difference between the formal and the actual charge. Formal charge is a bookkeeping tool that is important to help us keep track of the electron flow in the reaction.
The actual charge, however, is the actual electron density that is present on the atom. For instance, let’s take a look at borohydride anion:

The electronegativity of boron is 2.0 while electronegativity of hydrogen is 2.2. So, the hydrogen is more electronegative (not by much but still) and will polarize the bond. This means that hydrogen actually “pulls” the electron density towards itself. Thus:
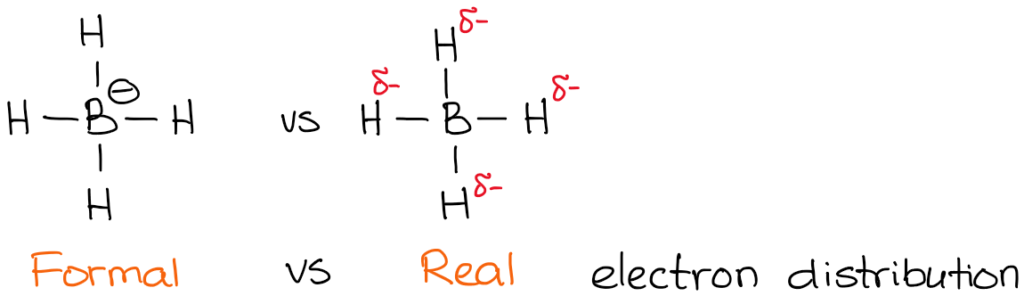
While formal charges are merely a “formality,” they are very important for the reactions mechanisms understanding. Thus you need to make sure you master the skill of quickly finding the formal charge.
You also notice that I’ve indicated my real electron densities with the delta-minus (𝛿-) symbol. That denotes that I only have a partial negative charge on each of the hydrogens. How much of that partial charge we have on them? Well, we could calculate it using fancy quantum chemical calculations, but that’s utterly unnecessary for the purposes of a typical organic chemistry course. What’s more important, is to realize that the boron is not actually negatively charged in this molecule. So, when we write a reaction with a borohydride anion, we won’t be showing electrons coming from boron!
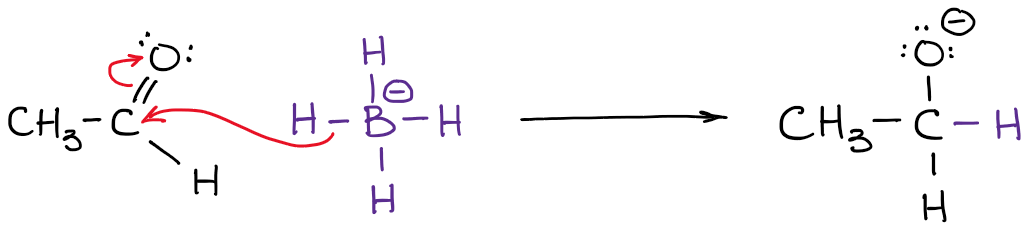
Notice how in the example above my arrow starts at the H-B bond and not at the boron atom! That’s because it’s not the boron that is a source of electron density here! You remember from earlier in this post, it’s the hydrogens are the atoms with the 𝛿-. So now it makes sense that the arrow doesn’t start at boron.

I don’t understand the comment at 2:45 in the video. Is the instructor saying that positive charged heteroatoms generally do not exist because they are unstable?
Atoms with open shells are very unstable and we don’t generally see them within the scope of organic molecules. The only open-shell positive species we’ll typically encounter are carbocations. But atoms like N, O, S, etc. are too unstable with only 6 electrons, so we’re not going to see them in organic chemistry.