Fischer Esterification
Fischer esterification is one of the must-know reactions of carboxylic acids. Reaction is named after Emil Fischer, one of the first Nobel Laureates who got 1902 Nobel Prize in chemistry in recognition of the extraordinary services he has rendered by his work on sugar and purine syntheses. The esterification is a reaction that makes esters from alcohols and carboxylic acids.
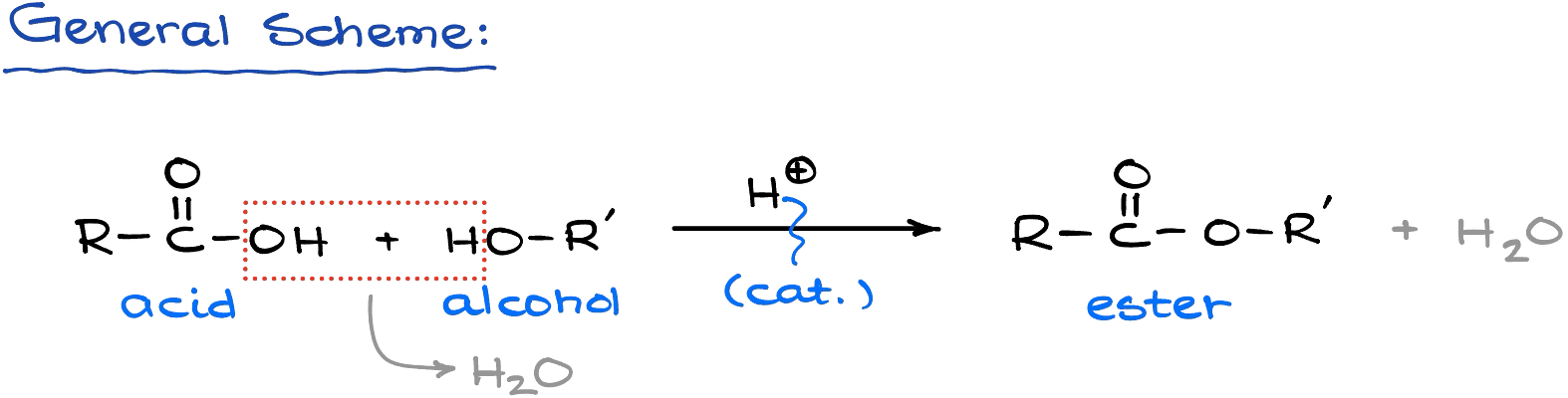
We need an acid to catalyze the reaction. Since carboxylic acids are not very electrophilic and alcohols are not particularly nucleophilic, addition of a strong acid helps increase the electrophilicity of the carboxylic acid.
Mechanism of the Fischer Esterification
Mechanistically speaking, Fischer esterification is a fairly typical acyl substitution reaction.
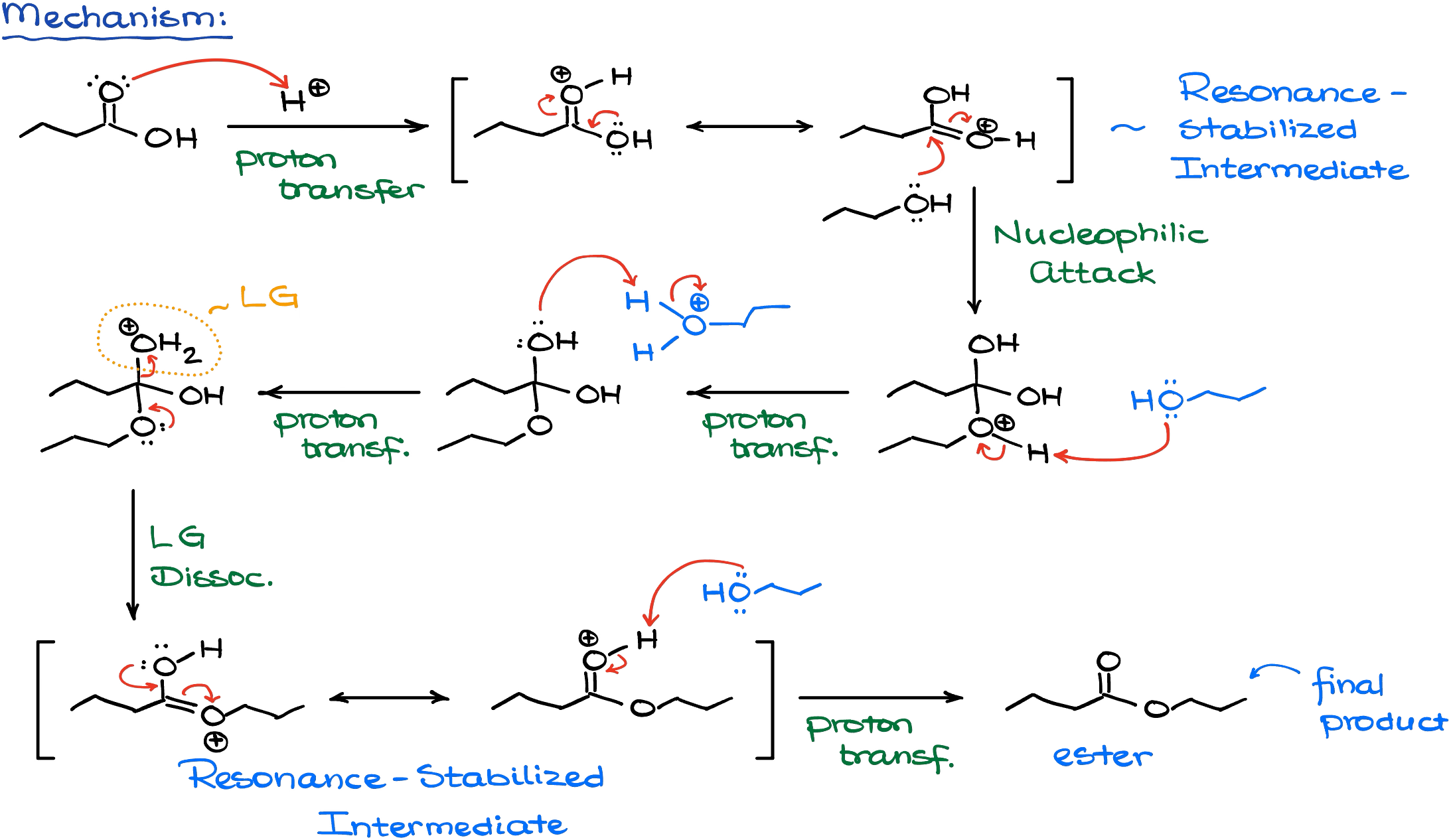
As I’ve mentioned earlier, reaction starts by making a better electrophile from our carboxylic acid by protonating it. An important thing to remember here is that we are always going to be protonating the oxygen of the carbonyl. This gives us a resonance-stabilized protonated species which is fairly electrophilic.
Once we have our electrophile, we’ll perform a nucleophilic attack from the alcohol. This gives us a tetrahedral intermediate. After a couple of proton transfers and a living group dissociation, we get our final product.
Fischer Esterification is an Equilibrium
Fischer esterification is an equilibrium reaction! Which means that technically each step needs to be shown with an equilibrium arrow. If your instructor is very particular about it, make sure you’re drawing the equilibrium arrows for every step in this reaction instead of the single-headed arrows like the ones I’m showing here.
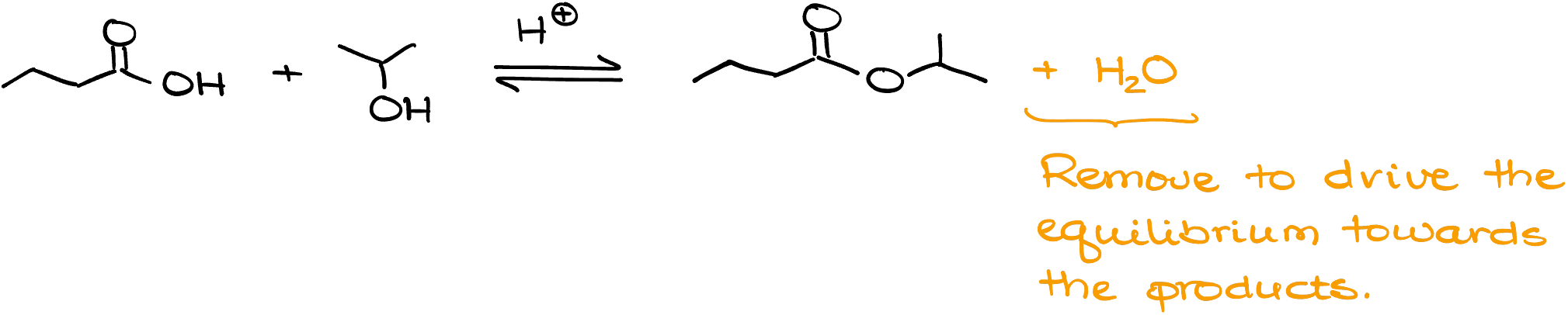
How do we increase the product yield? As in the case with any equilibrium, we can use the Le Chatelier’s principle by either increasing the concentration of one of our reagents, or removing one of our products. Adding extra reagents to your reaction mixture might not be the most efficient method, especially, if your starting materials are costly or hard to make. So, a more efficient method is removal of one of the products. Typically, we’re going to be removing water by either using the azeotropic distillation or 4Å molecular sieves that can physically remove water from the equilibrium.
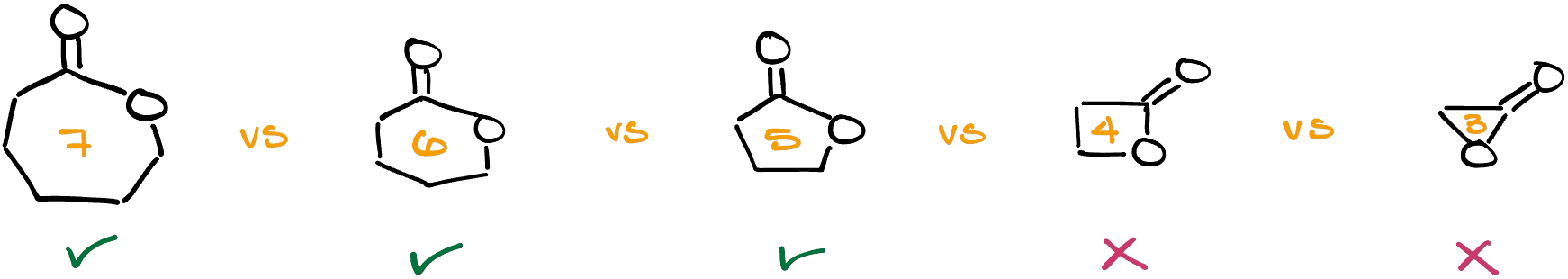
Intramolecular Fischer Esterification
Another interesting version of the Fischer esterification is the intramolecular version of the reaction yielding cyclic ethers (lactones).
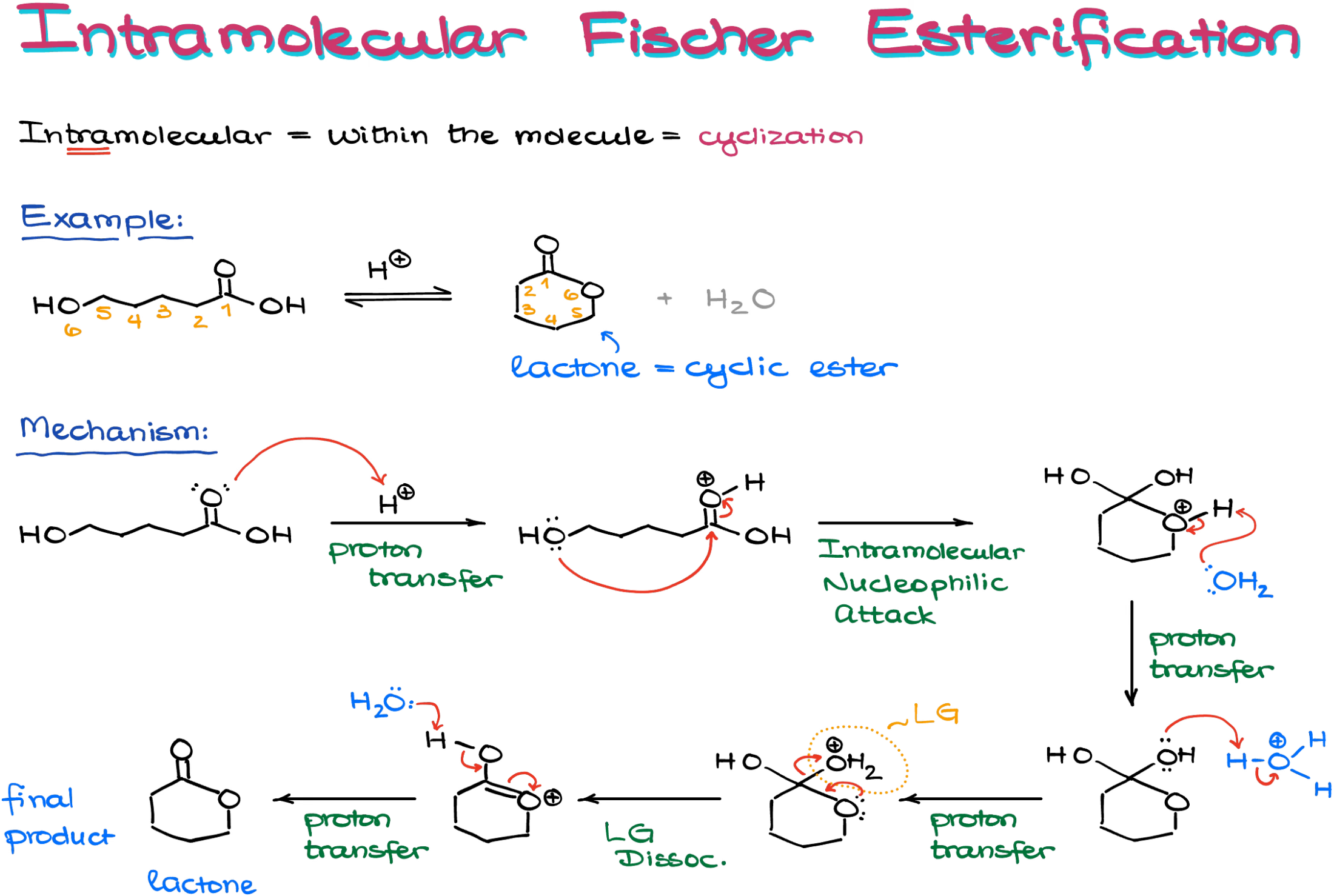
Keep in mind though, that this reaction won’t give you smaller rings like 3- or 4-membered lactones. But if you are making a 5- or a 6-membered ring, it’s a fair game.
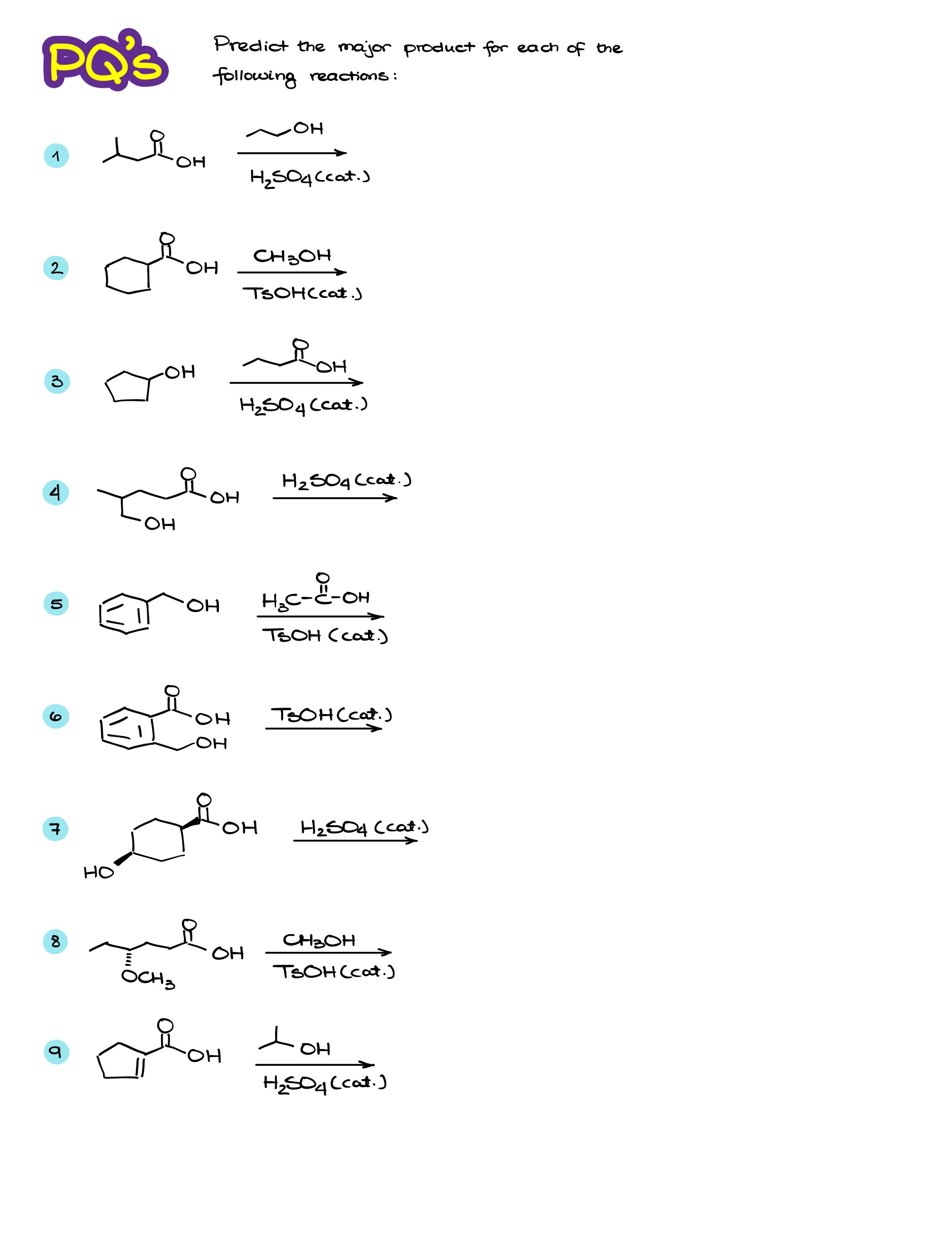
Practice Questions
Would you like to check your answers? Become a member or login.
