Epoxidation of Alkenes
Epoxidation of Alkenes is an important reaction for synthetic chemists and a frequent topic on exams in organic chemistry courses. The transformation to an epoxide functional group can be straightforward, yet it comes with nuances that can pose challenges, particularly when it comes to understanding its mechanism and the intricacies of the curved-arrow notation involved. My goal in this tutorial is to demystify these aspects and provide you with a comprehensive understanding of the epoxidation process. So, let’s dive in to explore the underlying principles, mechanism, and applications of this fascinating reaction.
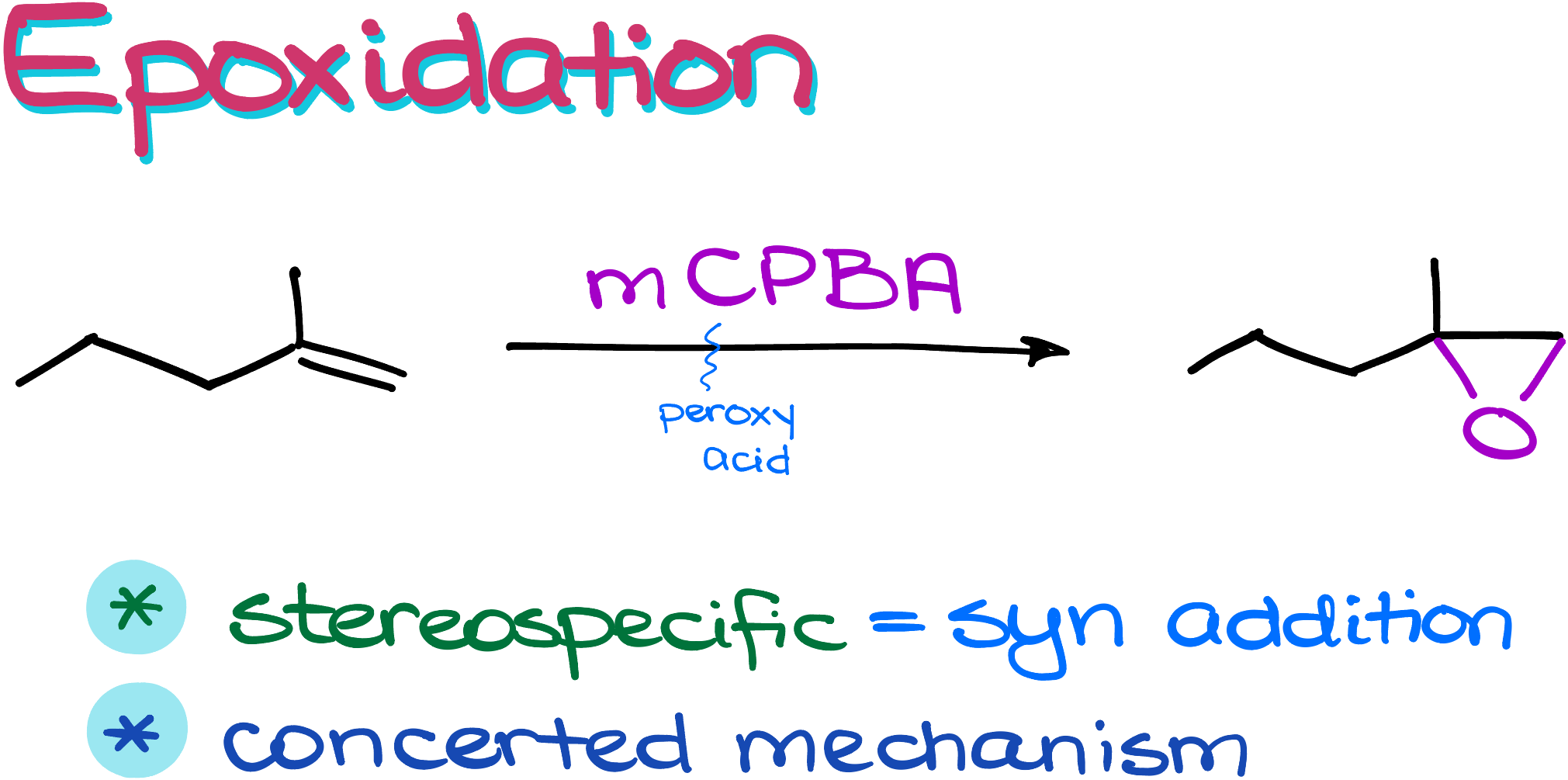
Mechanism
In the epoxidation mechanism, we start with an alkene that reacts with a peroxy acid. For visualization purposes, we can redraw the peroxy acid with one of the oxygens facing the alkene to make it easier to follow the mechanism.

The mechanism is initiated when the carbonyl group of the peroxy acid captures a proton. This is the first arrow in the sequence and sets the stage for the reaction.
Subsequently, the alkene attacks one of the carbons involved in the carbonyl group of the peroxy acid. In this step, the electrons from the alkene form the first carbon-oxygen (C-O) bond.
Then, the pi-electrons of the alkene loop back to attack the peroxy acid. This action establishes the second C-O bond, effectively forming the epoxide ring.
The final step involves breaking the oxygen-oxygen (O-O) bond in the peroxy acid, leading to the formation of the epoxide and a carboxylic acid as a by-product.
It’s crucial to note that this is a concerted mechanism, meaning that these steps occur simultaneously in one smooth, concerted action. While there are variations to this mechanism—some with four arrows and others with five—the essence remains the same. The four-arrow version is particularly favored for its simplicity and clarity.
Peroxy Acids
In the realm of epoxidation, me most commonly see three peroxy acids. Each has its unique set of attributes, yet for the purpose of this reaction, they can often be used interchangeably.

First on the list is mCPBA, short for meta-chloroperoxybenzoic acid. This is perhaps the peroxy acid we use most commonly.
Next, we have peroxyacetic acid. It is a liquid, unlike mCPBA, so it’s a little more difficult to handle and weigh out. But it’s a popular enough choice, so you will likely see it in your course.
Last but not least, there’s MMPP, which stands for magnesium monoperoxyphthalate. Like the others, MMPP serves as a capable epoxidizing agent and is used in various applications. While it’s not as abundant as the other two, some instructors like it as well.
Each of these peroxy acids comes with its own set of pros and cons, from reactivity to odor and the physical state. However, for the most part, they are interchangeable in the epoxidation of alkenes. In educational contexts, mCPBA is most often the go-to choice, but the other options are just as valid depending on your specific needs and preferences.
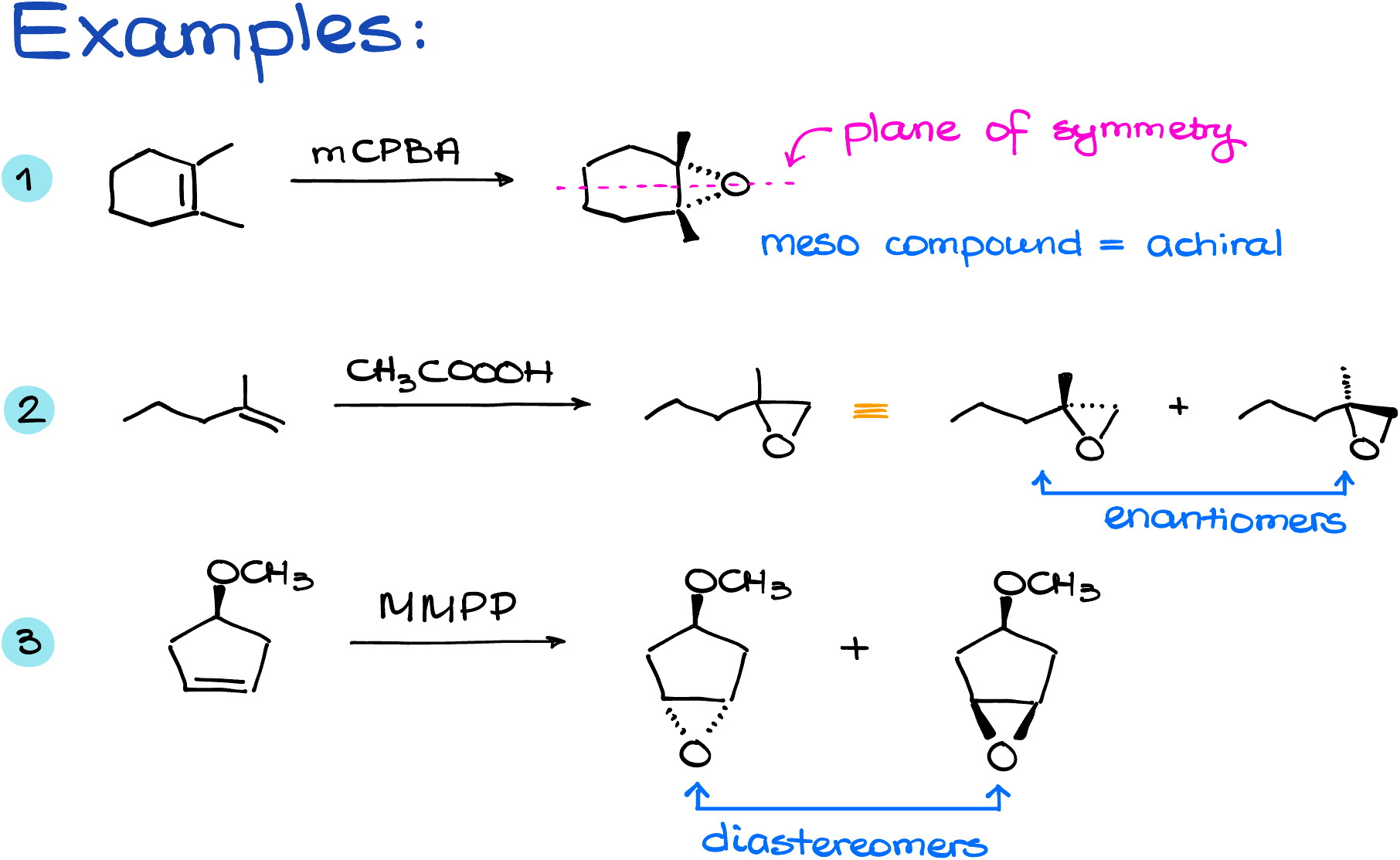
Examples
Here are a few examples to illustrate the epoxidation of alkenes reaction.
Recap
To summarize what we’ve covered, epoxidation transforms alkenes into epoxides. The reaction is stereospecific and it falls under the category of syn addition. The stereoconfiguration of the original alkene is crucial; if you start with a cis alkene, you’ll obtain one product, a meso compound in this example:

On the other hand, starting with a trans alkene, like trans-stilbene, results in a pair of enantiomers. These different outcomes underline the importance of the starting material’s stereoconfiguration in determining the product’s structure.
For carrying out the epoxidation, three peroxy acids are commonly used: mCPBA, MMPP, and peroxyacetic acid—each with its own set of advantages and disadvantages.
With this understanding, you are well-equipped to excel in any exam that includes questions on epoxidation reactions.
