Electrophilic Aromatic Substitution (Halogenation, Nitration, Sulfonation)
In this tutorial I want to talk about the electrophilic aromatic substitution (EAS) reactions. The EAS reactions are one of the fundamental and unique reactions of aromatic compounds. And in a typical organic chemistry course we cover five of them.
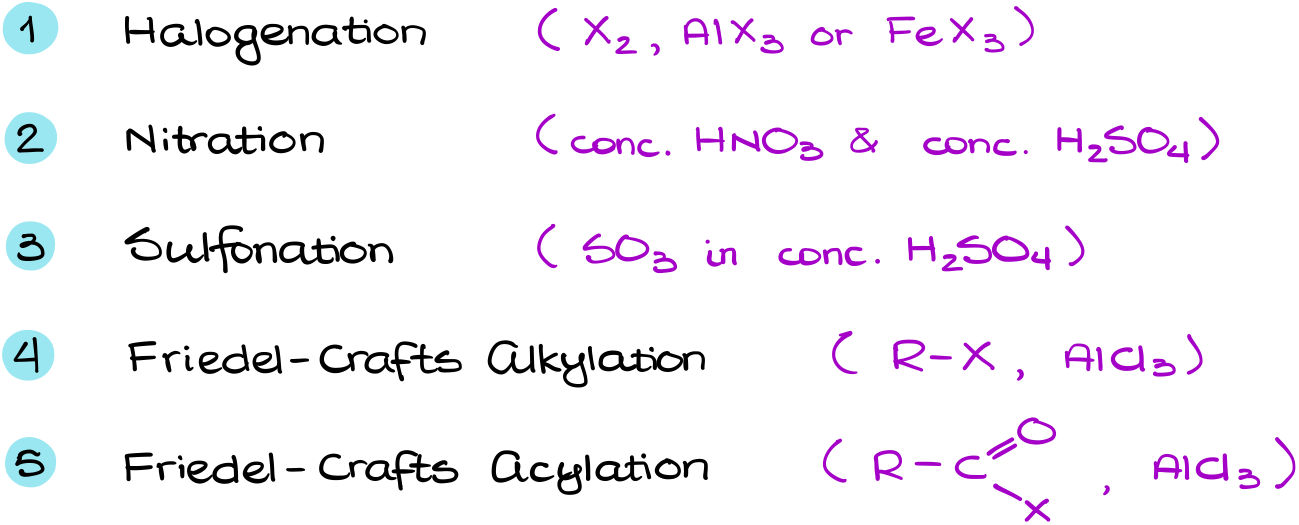
Generic Mechanism for the Electrophilic Aromatic Substitution
So, let’s look at the generic mechanism for the electrophilic aromatic substitution reactions.
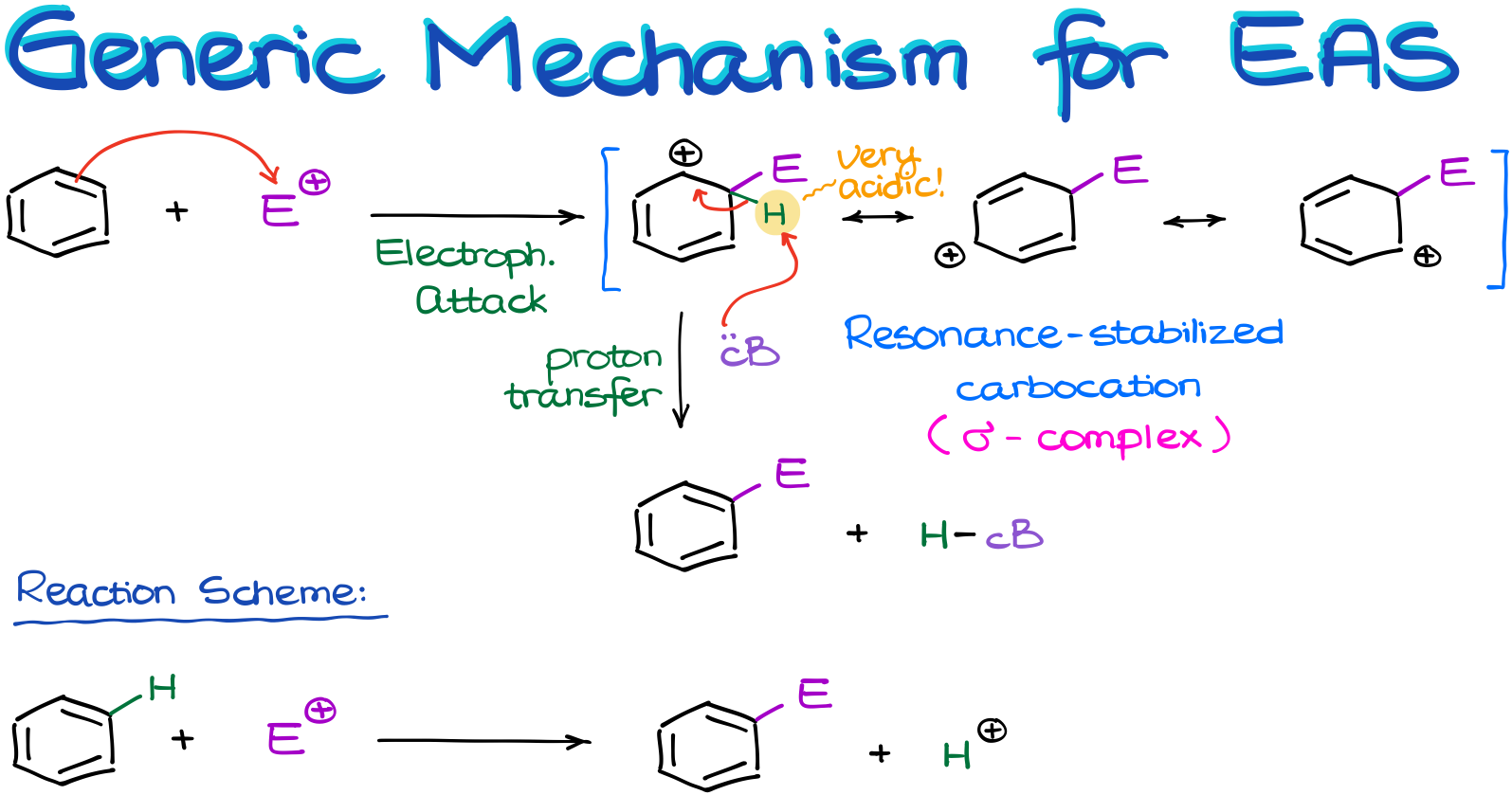
Let’s start by reacting the benzene molecule with some sort of electrophile. The nature of the electrophile is irrelevant for the moment. The only thing we need to know about our electrophile is that it’s a strong one. And the reason why we need a strong electrophile is because aromatic compounds are very stable. So, if we want to attack the π-system and break aromaticity, we’d need something very strong to do that.
And once that happens, we are going to end up with a carbocation. This carbocation is resonance stabilized, so we can draw three resonance contributors for it. There will be a lot of resonance drawings in this topic. So, if you’re not feeling very comfortable with the resonance, or need a refresher, make sure you review it now. We’re going to be using resonance as one of our main tools of investigation here, so it’s crucial you’re capable of using it.
Now, back to our resonance-stabilized carbocation.
We occasionally call this intermediate a “σ-complex.”
The σ-complex is an outdated term. But it was very popular in the middle of the last century. And you can still see in in some textbooks up to this date.
So, once we have our carbocation, we have a bit of a problem on our hands. You see, to make this carbocation, we had to break the aromatic ring. And thus, we’ve lost the stability that comes with it. And as I’ve mentioned in my previous tutorial on aromaticity, molecules will do whatever it takes to regain aromaticity or to maintain it. This intermediate is no exception.
But what are going to do to make our molecule aromatic again? Well, we can lose the proton from the position where we’ve attached the electrophile!
The highlighted proton (in the scheme above) is incredibly acidic. It’s a proton on a carbocation and we know that carbocations are wickedly acidic. Also, the removal of this proton restores aromaticity. So, it will be a very thermodynamically favorable process if we were to get rid of it.
So, some sort of a conjugate base—again, the nature of the base is irrelevant for the moment—will come and pull it off. Due to how acidic that proton is, we don’t even need anything very basic. Essentially, anything with an electron pair will do.
Here is an important thing I want to point out before we move further. While this reaction might look like some sort of addition because we just “added” an electrophile to the molecule, it is not.
If you pay close attention to what’s been going on with the reagents, you can see that there was a hydrogen on the aromatic ring. And in the course of this reaction, we’ve replaced that hydrogen with an electrophile. So, while we don’t normally consider hydrogens as leaving groups, in this reaction it, essentially, plays a role of one by being replaced with an electrophile. Therefore, this is a substitution reaction. Although, it may not look like one, especially, if we’re writing the reaction using the skeletal structures without showing the implicit hydrogen atoms.
Alright. Now, let’s look at the actual electrophilic aromatic substitution reactions I’ve mentioned at the beginning of this tutorial.
Halogenation of Aromatic Compounds
And we’ll start with the halogenation reaction.
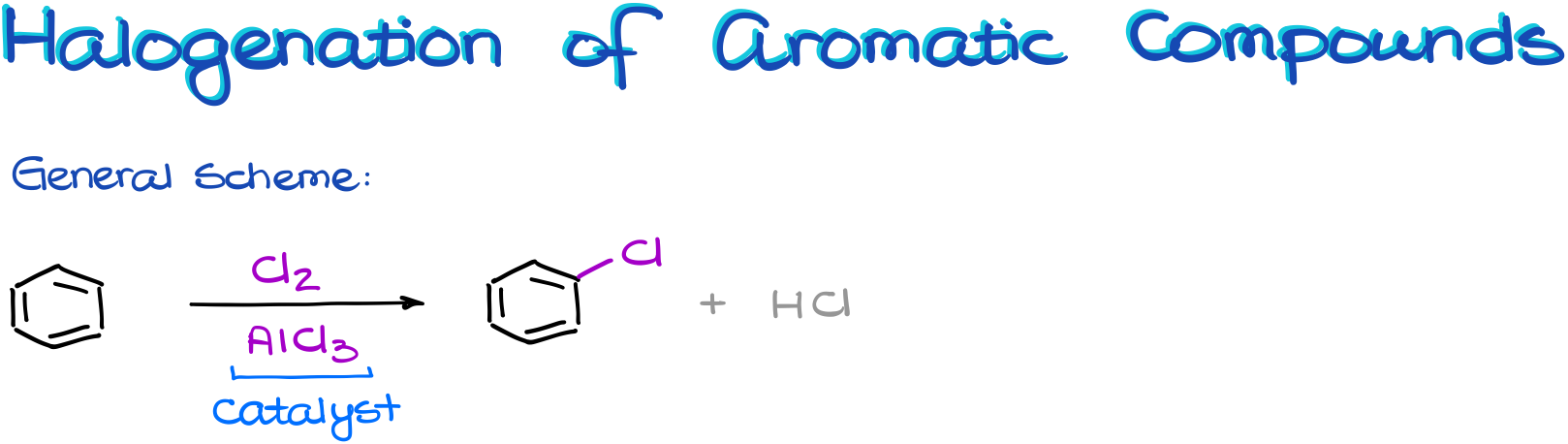
In the general scheme, we see that we need a halogen, I’ll be using chlorine here, and another species, aluminum chloride.
Aluminum chloride is the catalyst that we’ll need to make a good electrophile. And as a catalyst, it’s not going to be consumed in this reaction. So, once we start working through the mechanism of this reaction, we’ll have to make sure we regenerate it.
Interestingly enough: although we’re used to think about catalysts as something we use in a very small quantity, in the electrophilic aromatic substitution reactions we’re actually going to be using almost equimolar quantity of our catalyst. Meaning, we’ll be using the one-to-one ratio of our catalyst to the starting materials. So, if you ever do this reaction in the lab, don’t get surprised by the amount of the aluminum halide the procedure asks for. It’s gonna be a ton instead of our typical “tip of the spatula” deal.
Anyways, cute factoids aside, let’s get back to the mechanism of this reaction.
The first step in this reaction is going to be the formation of our electrophile.
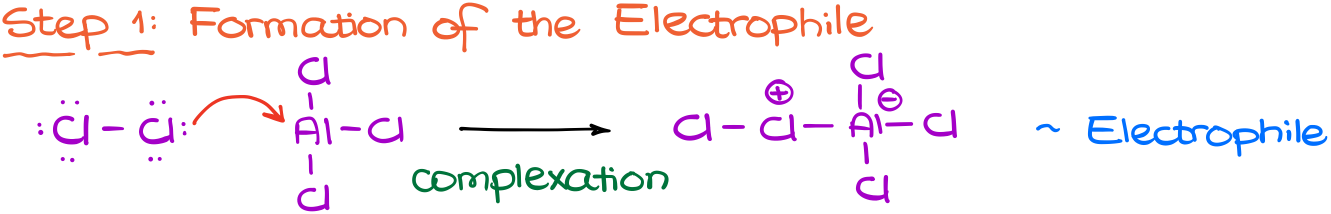
While chlorine is electrophilic, it’s not electrophilic enough to tackle the aromatic ring and disrupt aromaticity.
So, this is where the aluminum chloride comes in. Many metal halides are good Lewis Acids, meaning they are good at accepting electrons. Aluminum chloride is an exceptional Lewis Acid. So, it will pull the chlorine towards itself to steal some of the chlorine’s electron density.
This will yield a new complex between the chlorine molecule and aluminum. And because chlorine has to provide some of its own electron density to make this bond, chlorine becomes very electrophilic.
Now, this new complex is going to be our electrophile.
The next step in going to be our electrophilic attack.
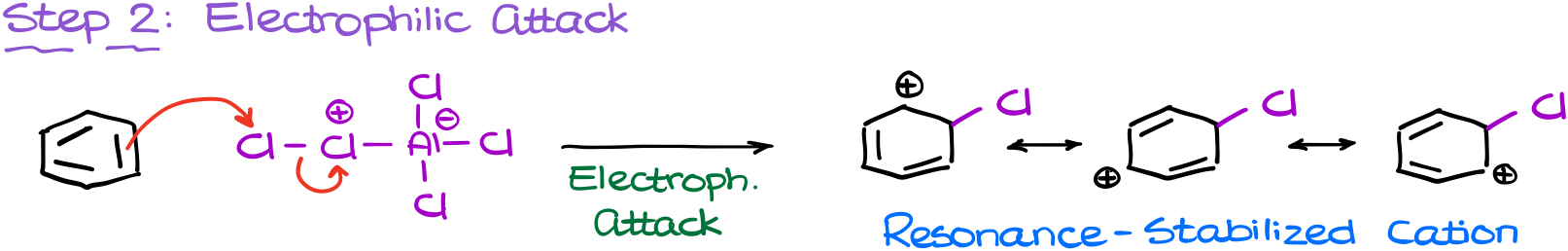
In this step our newly made electrophile attacks the aromatic ring. Notice, that in this attack, it’s the chlorin atom on the edge of the molecule that’s actually doing the attacking and not the one with a formal positive charge.
This attack results in the formation of our resonance-stabilized carbocation intermediate, the σ-complex.
And finally, we’re going to restore the aromaticity by removing the proton from our carbocationic intermediate. As a conjugate base we are going to use the “leftover” of our catalyst that’s now has an extra chlorine atom.
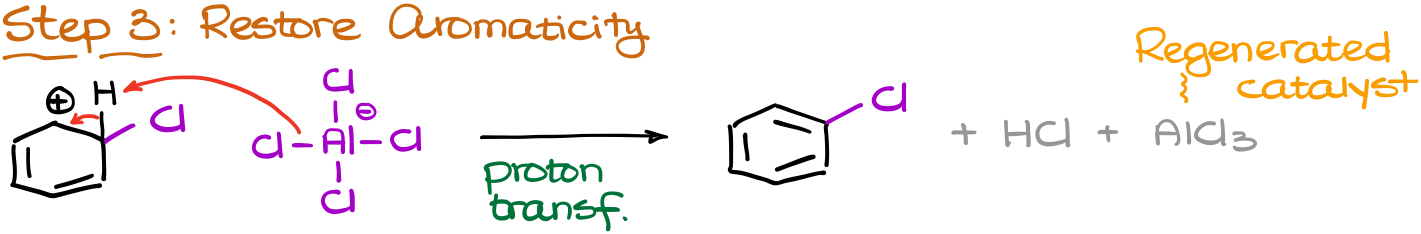
That gives us the final product, chlorobenzene, and the regenerated catalyst along with the HCl co-product.
I also want to mention that this reaction only works for chlorination and bromination. We don’t normally do this reaction for fluorine as those are difficult to control, not to mention that fluorine is extremely dangerous and a nightmare to handle. And iodine is too unreactive even in the presence of the Lewis Acid catalysts. There are methods allowing us to do the direct fluorination and iodination, but those are a bit of chemical exotics and I’ll talk about those in a different post.
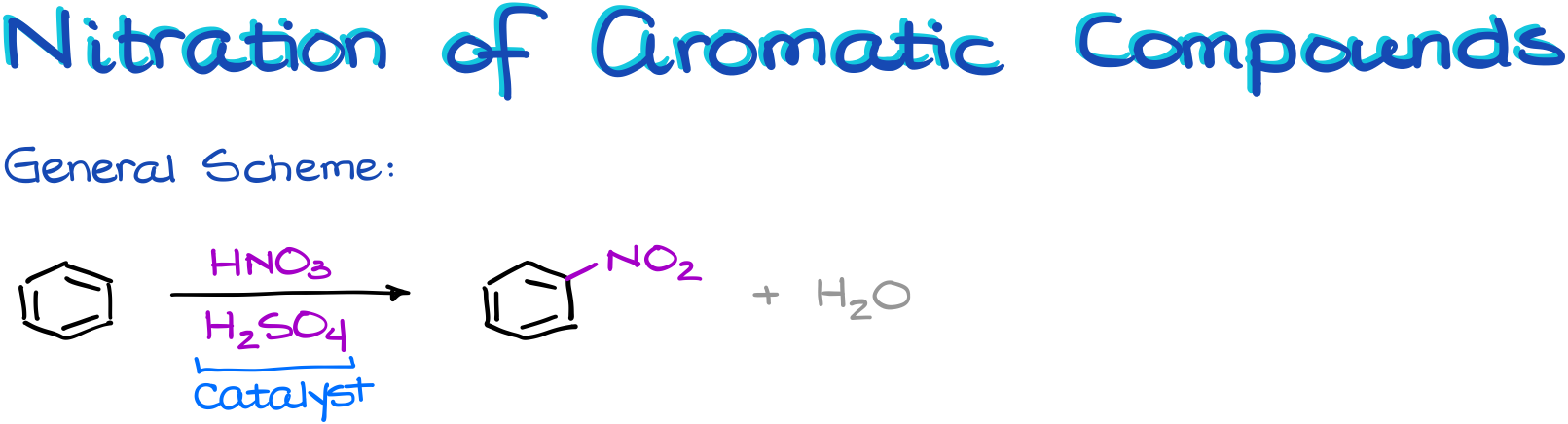
Nitration of Aromatic Compounds
In the general scheme we can see again that we have a main reagent, the nitric acid, and a catalyst, sulfuric acid. Like in the previous case, the nitric acid is not electrophilic enough by itself and it cannot tackle the aromatic ring as is. So, it needs a little help to become more electrophilic.
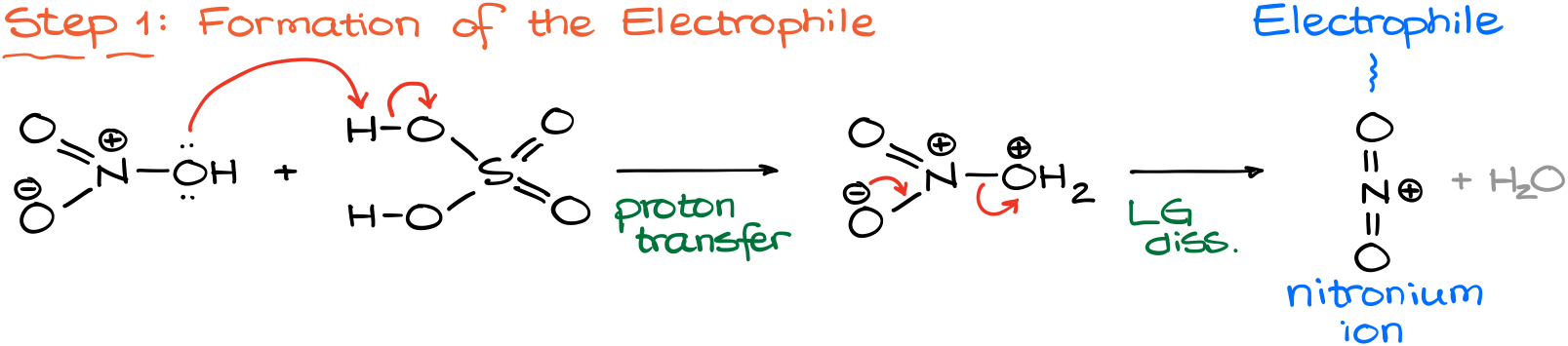
As sulfuric acid is a stronger acid than the nitric acid, the reaction starts by a proton transfer from sulfuric acid to the oxygen of nitric acid. This forms a rather unstable protonated nitric acid, which quickly undergoes the leaving group dissociation giving us the nitronium ion, which is our electrophile.
And as the nitronium ion is a strong electrophile, it’ll have no problems attacking the aromatic ring making a resonance-stabilized carbocation.
I’m not going to show the full Lewis structure of the nitro group here for the sake of space. But I encourage you to draw that on the exam in its full glory as many instructors will appreciate the fact that you actually know how the nitro group looks like. Jokes aside, but you have no idea how common of an error it is. For whatever reason, students just don’t remember the structure of the nitro group.
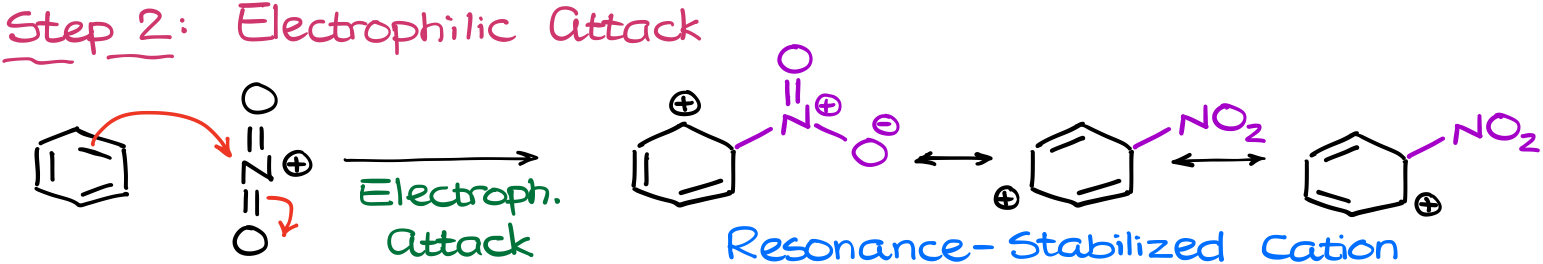
Okay, I’ll stop complaining and let’s move to the next step.
And once we have our carbocation intermediate, the last step in this reaction is the aromaticity restoration via the proton transfer like in the previous case.

Here, we’re going to use the conjugate base “leftover” from the sulfuric acid regenerating our catalyst and giving us the final product.
Nitration is a very aggressive reaction, and we never want to perform it on a sensitive and a very complicated molecule. The nitric and sulfuric acids together can “eat” through just about anything, so many functional groups may be destroyed in this reaction if we are not careful.
Sulfonation of Aromatic Compounds
In this reaction we are going to treat our aromatic molecule with the fuming sulfuric acid, which is a solution of the SO3 in concentrated sulfuric acid. This mixture is a thick viscous yellow-brown liquid, and because of that it is sometimes called the “oleum” acid.
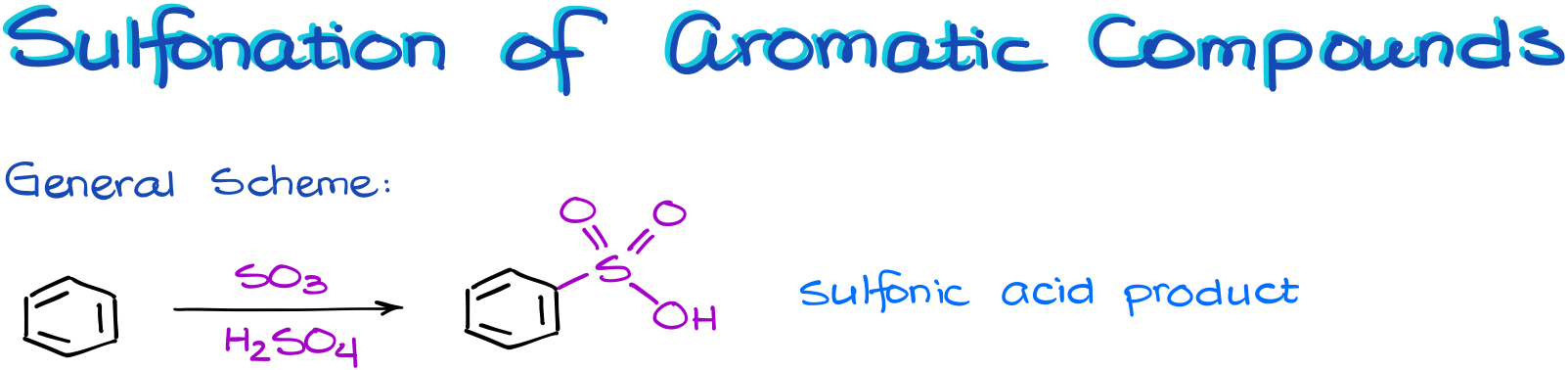
This reaction results in a sulfonic acid. And interestingly, there are no co-products in this reaction.
As always, we are going to start our mechanism with the electrophile formation. In this case, it’s going to be the protonation of the SO3 by sulfuric acid.
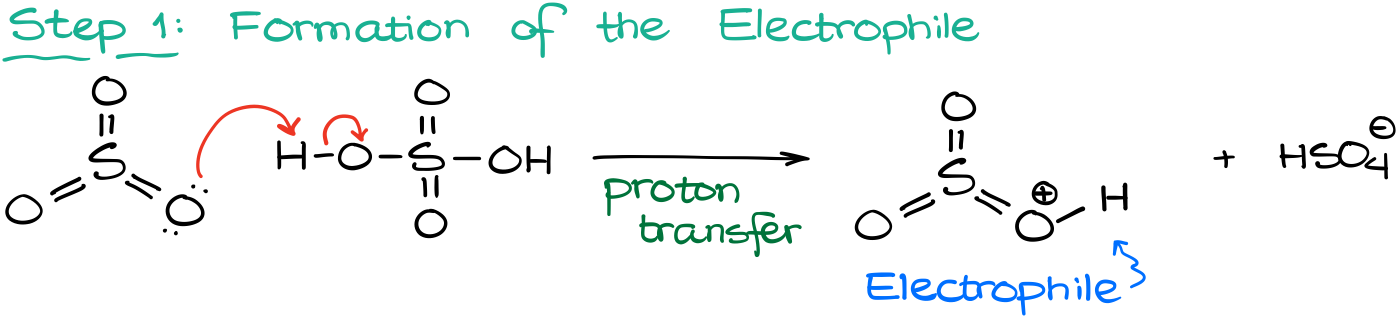
This gives us the electrophile that will then attack the aromatic ring. Again, here the SO3 by itself is just not strong enough to attack the aromatic ring, so we need to take it over the edge by protonating it.
The electrophilic attack in this reaction gives us our typical carbocation intermediate. As this is already the third time, we’re seeing the same style intermediate in this tutorial, I’m not going to show all the resonance structures. So, try drawing them out and see if you can do it on your own. You can use the other two examples as a template for your resonance structures.
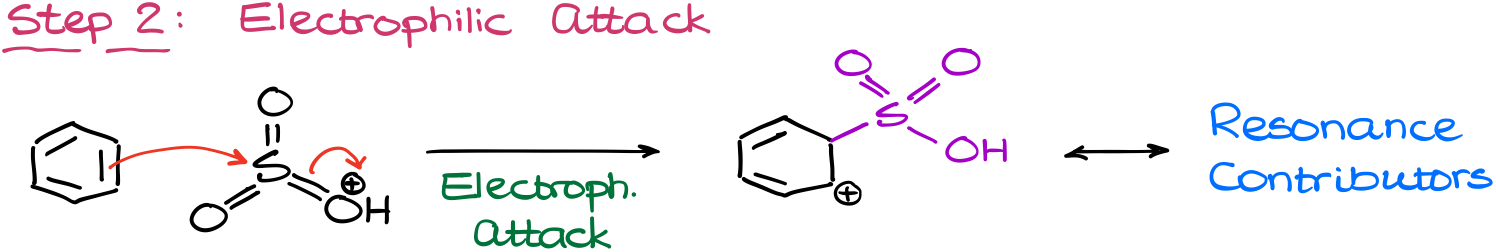
Finally, we are going to restore aromaticity by removing the proton from our carbocation. In this reaction, we’re again going to be using the sulfuric acid conjugate base for these purposes.
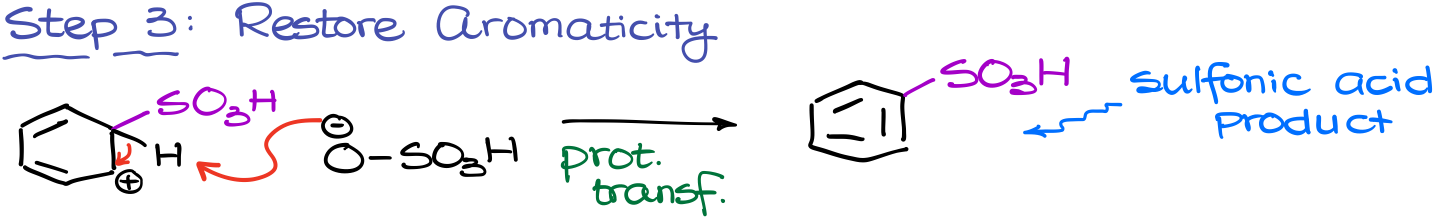
This yields the final product: sulfonic acid.
Here’s something really interesting about this reaction: it’s reversible. This means that we can also remove the sulfonic acid group if we do this reaction in a dilute sulfuric acid solution. As a challenge, I encourage you to come up with the mechanism of this reaction using all the same steps in the reverse order.
Why is this important to know? Well, later in your course you’ll start talking more about the multistep synthesis. And this reaction becomes a useful “trick” which you can use to temporarily “block” the position in the aromatic ring, do some other chemistry, and then remove this temporary “block” when you no longer need it.
Review of Halogenation, Nitration, and Sulfonation of Aromatic Compounds
Let’s do a quick review of what we’ve just learned.
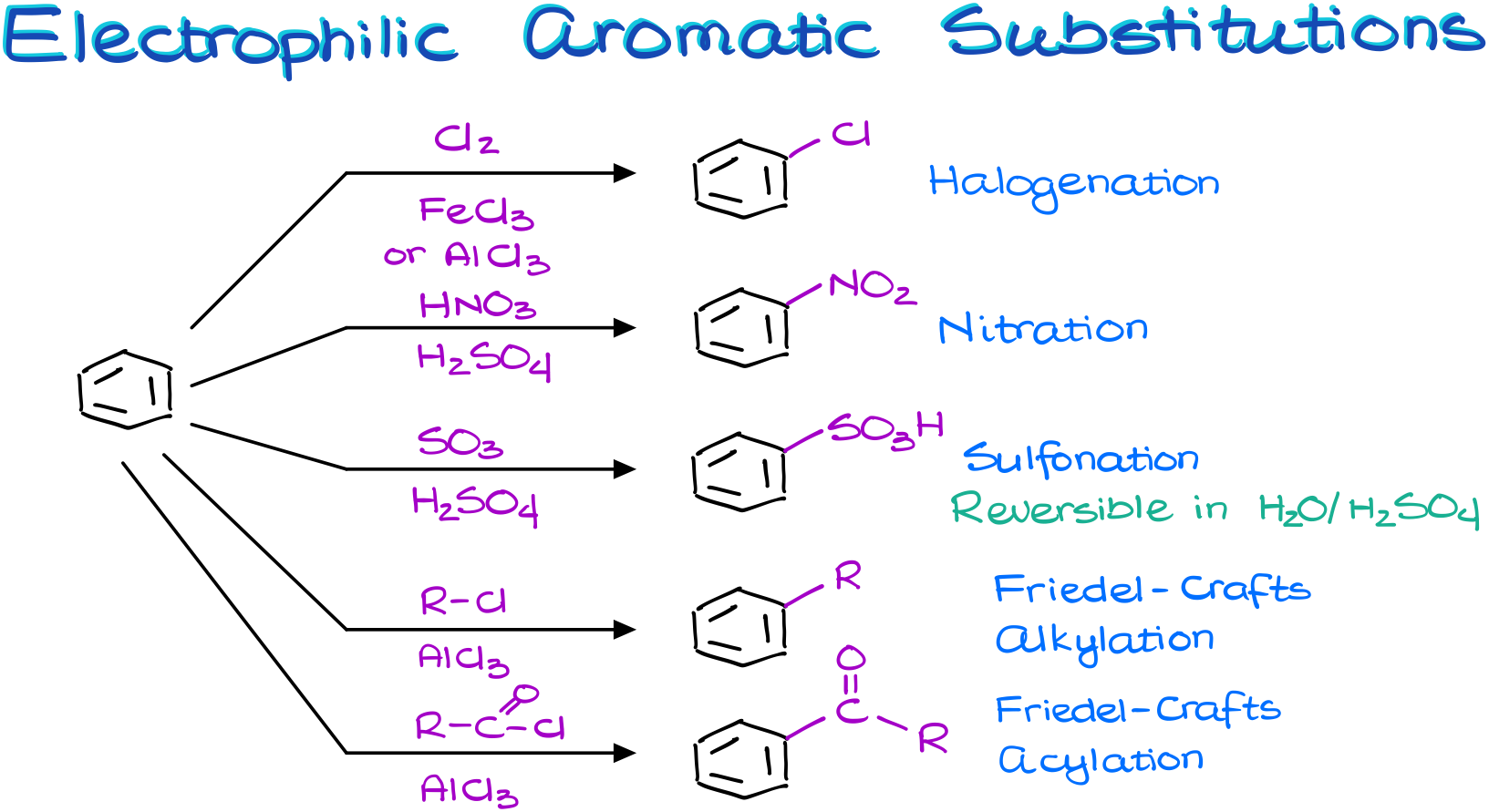
If we treat an aromatic compound with a halogen (either a chlorine or a bromine) in the presence of the Lewis Acid catalyst, we get the halogenation reaction.
If we treat an aromatic compound with a nitrating mixture, which is a mixture of concentrated nitric and sulfuric acids, we get a nitrated aromatic compound. And we call this reaction the nitration.
The treatment of aromatic compounds with the fuming sulfuric acid results in the sulfonation reaction. And I’m going to remind you here again that this is a reversible reaction. So, we can take the sulfonic acid group off our molecule in the diluted sulfuric acid.
Finally, we have the Friedel-Crafts alkylation and acylation. I haven’t talked about those two reactions in this vide for a very good reason. They are much more complicated and have more extremely important details we’ll need to go over. So, these reactions deserve their own tutorial.
