Electrophiles and Nucleophiles
In in this post I want to look at the difference between the nucleophiles and electrophiles, what those are, how to identify them in a reaction, and some common examples you’re going to see in your organic chemistry course.
So, first off, let’s talk about what the nucleophiles and electrophiles are.
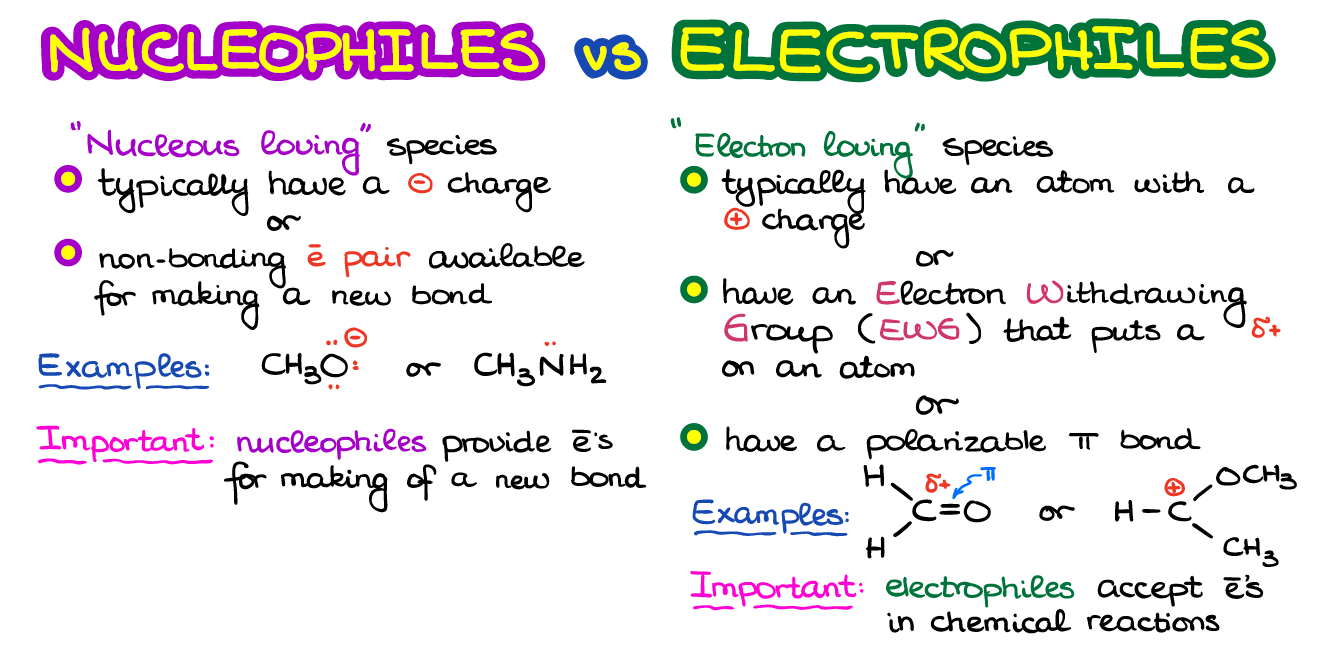
What is a Nucleophile
A nucleophile is a “nucleus loving” species if we look at the word itself and translate its Greek roots. The nucleophiles are typically negatively charged or have at least one electron pair they can easily share to make a new chemical bond.
For instance, the CH3O– and CH3NH2 are a couple of examples of common nucleophiles. In the first case, we have a negative charge. Negatively charged species have an excess of electron density, which means they can easily share some of those excess electrons with electron-deficient species making a new bond. Important thing to remember her is that nucleophiles will always play a role of electron donors in chemical reaction.
What is an Electrophile?
So, what about the electrophiles? Well, they are the complete opposite. They are the “electron loving” species and they are typically either positively charged or have a partial positive charge (δ+). In other words, electrophiles are electron-deficient species and are looking to get some more electrons from elsewhere. Electrophiles will often have electron-withdrawing groups (a group containing electronegative elements pulling the electron density towards themselves). Alternatively, electrophiles may also have polarizable π-bonds such as C=O or C=N.
For example, in the picture at the beginning of this post we have a couple of electrophilic molecules. The first one has a very polar C=O bond which puts an extremely high partial positive charge (δ+) on carbon. The second one is what we would call a carbocation—a species with 6 electrons around carbon. Since carbon does not have a complete octet around on the valence shell, it’s rather unstable and electrophilic.
An important thing to remember about the electrophiles is that they are going to be the acceptors of the electrons in a reaction. By accepting some electrons from nucleophiles, electrophiles will “quench” their positive or partial positive charge making a more overall stable species.
How to Find Nucleophiles and Electrophiles in a Reaction
So, now when we know what the nucleophiles and electrophiles are, let’s look at a few examples and try to find those in each reaction.
Example 1
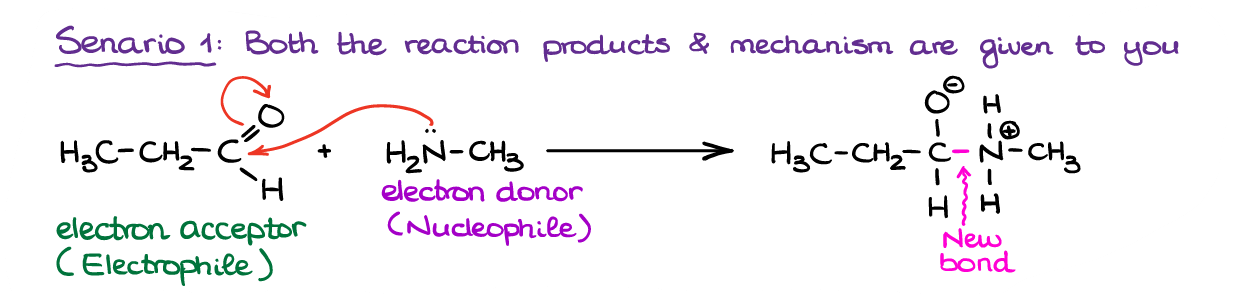
In this first scenario we have a reaction with the products and the mechanism already given to us. This makes our task much easier. We simply need to follow the electron flow from one species to another. Thus, we can see that the molecule on the left (and aldehyde) is an overall electron acceptor, while the molecule on the right (an amine) is an electron donor. This way, we can classify the aldehyde in this reaction as an electrophile and the amine as a nucleophile. Notice, by the way, how nitrogen provided the electrons for the new bond in the product.
Example 2
Here’s the second scenario.
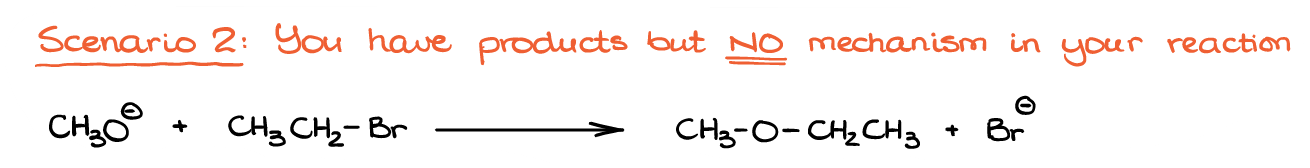
We have the reaction products, but we don’t have the curved arrow mechanism to show us the electron flow from one species to another, so we’ll have to figure that one for ourselves. So, the question is: how exactly are we going to do that?
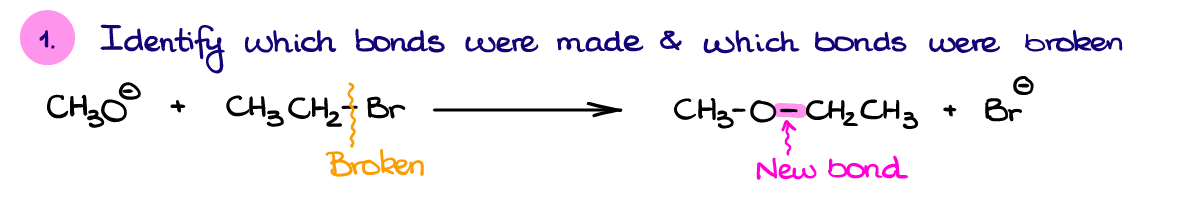
Well, first, let’s identify the bond made and bonds broken in this reaction. Based on the structure of our product here, we can see that we have made a new C-O bond. We also broke a C-Br bond since the Br– is a free species on the product side.
Next, we want to identify the electron flow in this reaction. In this case, it’s reasonable to assume that the negatively charged species is going to be our nucleophile since it has an excess of electron density. Generally, if you have a reaction between a negatively charged species and a neutral one, the negative ion will be the nucleophile. Likewise, if you’re dealing with a reaction between a neutral molecule and a positive ion (cation), then the neutral molecule will have a generally higher electron density and will act as a nucleophile. So, in this reaction, the negative oxygen is our nucleophilic piece, while the carbon attached to bromine is going to be an electrophile.
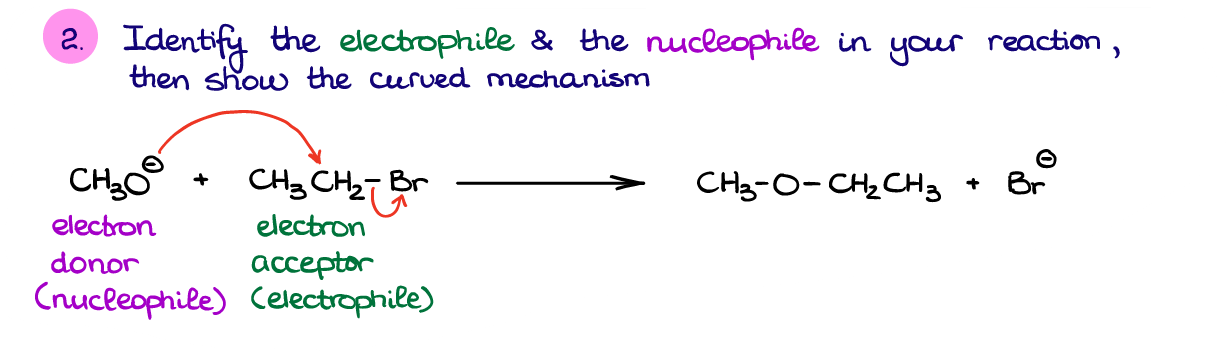
Remember, I knew that we are making the C-O bond, so since we’ve identified the O as a nucleophile, the corresponding C must be an electrophile. This is because you’ll always move electrons from a nucleophile to an electrophile to make a chemical bond. I know I’ve already mentioned that before, but I just want to make sure that this fundamental principle sticks in.
Example 3
Alright, how about the third scenario now where we only have the reagents. We don’t know the products or the mechanism, so we’ll have to figure out everything for ourselves. This is, perhaps, the more common type of an exam question, so you’re likely to see something like that on your exam or in your homework.

So, the first thing in figuring out what’s going on in this reaction is to find all the places with high electron density (δ- or electron pairs) and places with low electron density (δ+ or + charges). Identifying the electron pairs is fairly easy: check the element’s position in the periodic table, then see how many bonds it has, and add necessary electrons to complete the octet. Often, your instructor will already place all the electron pairs on the atoms in your molecules (especially early in the course).
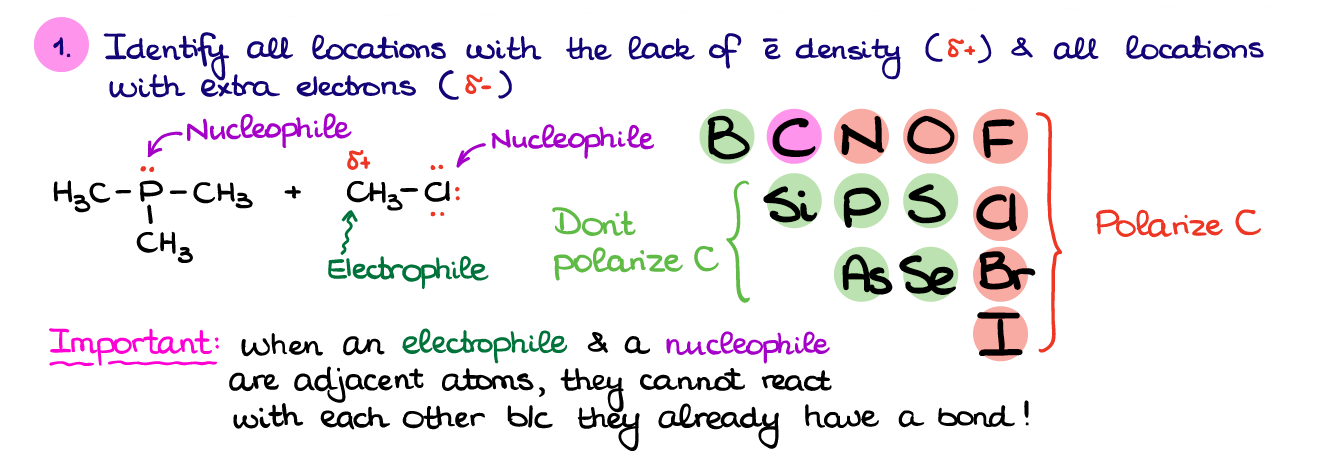
How are we going to deal with the partial charges though? Ideally, we’d need to look at the difference in electronegativity between carbon and other elements. Naturally, nobody expects you to remember the electronegativity values for all non-metals. There’s however, a simple trick.
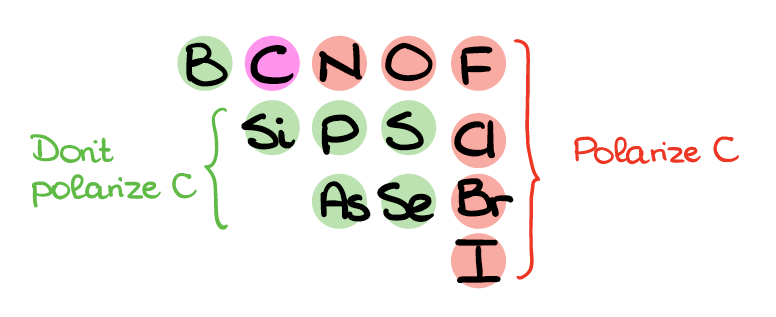
Here’s the part of periodic table with the non-metals you may see in organic molecules. The ones that I highlighted in red will polarize carbon and add δ+ on carbon. The green elements, however, won’t polarize carbon sufficiently, so those bonds won’t really do much for the electron density on C in most cases. So, when C is bonded to N, O, F, Cl, Br, or I, we can go ahead and place a δ+ on that carbon. However, if your C is bonded to any other non-metal, leave it as is.
After you’ve identified the places in your molecules with electron surplus and places with electron deficiency, we can assign the potential electrophiles and nucleophiles. And here’s something particularly important: when you have an adjacent nucleophile and an electrophile, you’ll have to choose just one. Since our left molecule is only a nucleophile, the right molecule, thus, will be an electrophile.
And once we’ve identified our nucleophile and electrophile, we can propose an electron flow from one molecule to another using curved arrows.
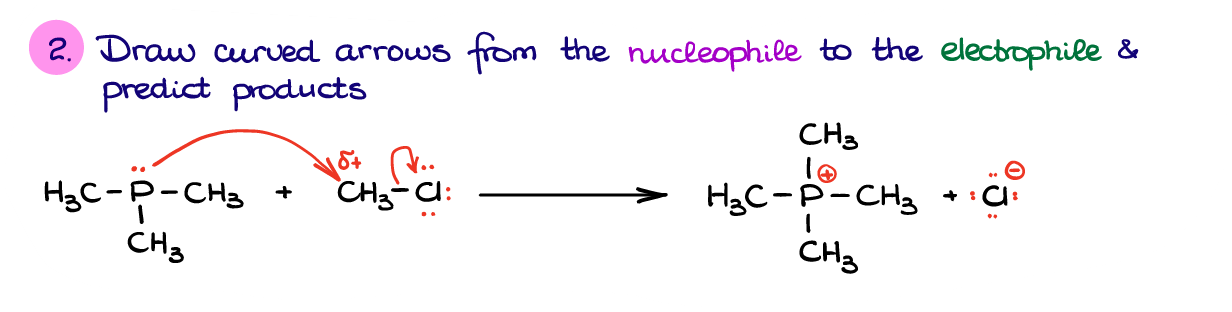
As nucleophile provides the electrons to the electrophile, we’re going to show the arrow from phosphorus to the carbon. However, if the carbon atom accepts those electrons, it will have way too many electrons on the outer shell. So, to accept those electrons from the nucleophile it will have to break one of its bonds. As a rule of thumb, you always want to break a bond to what we call a better “leaving group.” A leaving group is a species that is stable bearing a negative charge or becomes a neutral molecule upon dissociation. In this case we can either break a bond to Cl or to one of the H’s. If we compare the H– and Cl– as the two potential leaving groups, the Cl– is a much more stable ion. For the sake of time and staying on topic, we’ll discuss the ion stability and why exactly the Cl– is more stable than H– in another post. So, to recap what I just said, to accept the electrons from P, we’ll need to break the C-Cl bond.
Doing so, gives us the products in this reaction. And we’re done! Remember, practice makes perfect. Make sure you go through enough practice problems identifying nucleophiles and electrophiles various examples using these steps. You want to be able to identify the nucleophiles and electrophiles automatically by just looking at your molecules. Normally, this will be the first step in most of the reaction mechanisms, so you’ll be using this skill over and over again.
Typical Nucleophiles and Electrophiles
And while it is important to know the steps in identifying the nucleophiles and electrophiles in reactions, most of the time, you’re going to see a lot of similarities from one molecule to the other. At the end of the day, organic chemistry is a science of patterns. And the whole ordeal with electrophiles and nucleophiles is not different.
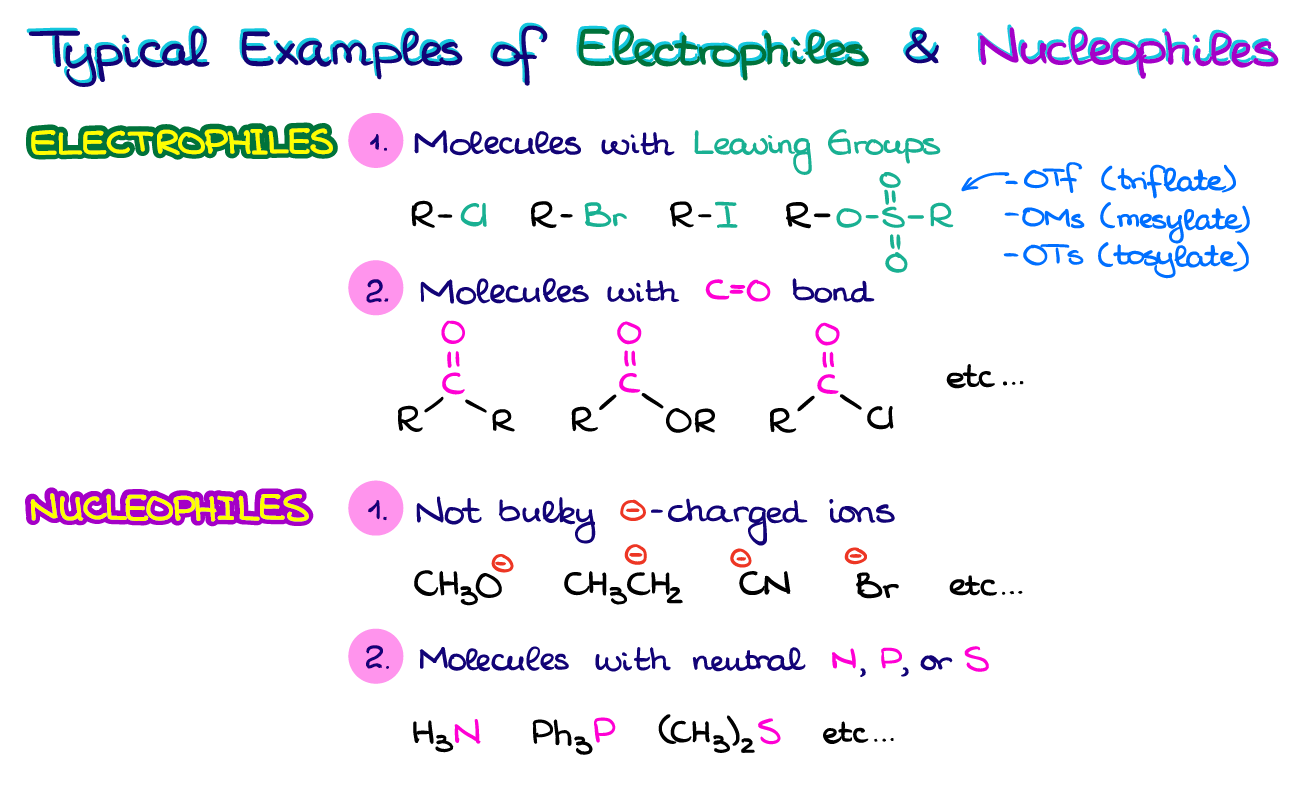
Here I have some examples of the typical electrophiles and nucleophiles you’re going to see in your course. Your typical electrophiles will have good leaving groups like halides or sulfonate ester groups. They may also have polarizable C=O bonds like in aldehydes, ketones, or carboxylic acids derivatives. When it comes to nucleophiles, those are going to be either some smaller negatively charged species or molecules with N, P, or S atoms. While there are many examples of electrophiles and nucleophiles out there and it’s just impossible to summarize them all in one table, these tend to pop up most often.
