E2 Reactions
In this tutorial, we are going to talk about the E2 reactions, which is, probably, the best type of elimination reactions out there because it gives chemists a lot of control over the reaction outcomes.
So, the goal of the E2 reaction like any other elimination reaction out there is to rip something off our molecule and make a new π-bond.
E2 is a bimolecular reaction. So, similarly to the SN2 reaction, the rate of the reaction depends on both concentrations of the substrate and the base. However, since it’s an elimination and not a substitution reaction, we’re going to typically compare the E2 reactions to E1 reactions.
The big difference between E1 and E2 is that E2 reactions have the concerted mechanism, just like the SN2 reactions. So, we are not going to form any intermediates in this reaction, and everything will happen in one step. Thus, if I have some sort of generic molecule here, the way this reaction works is:
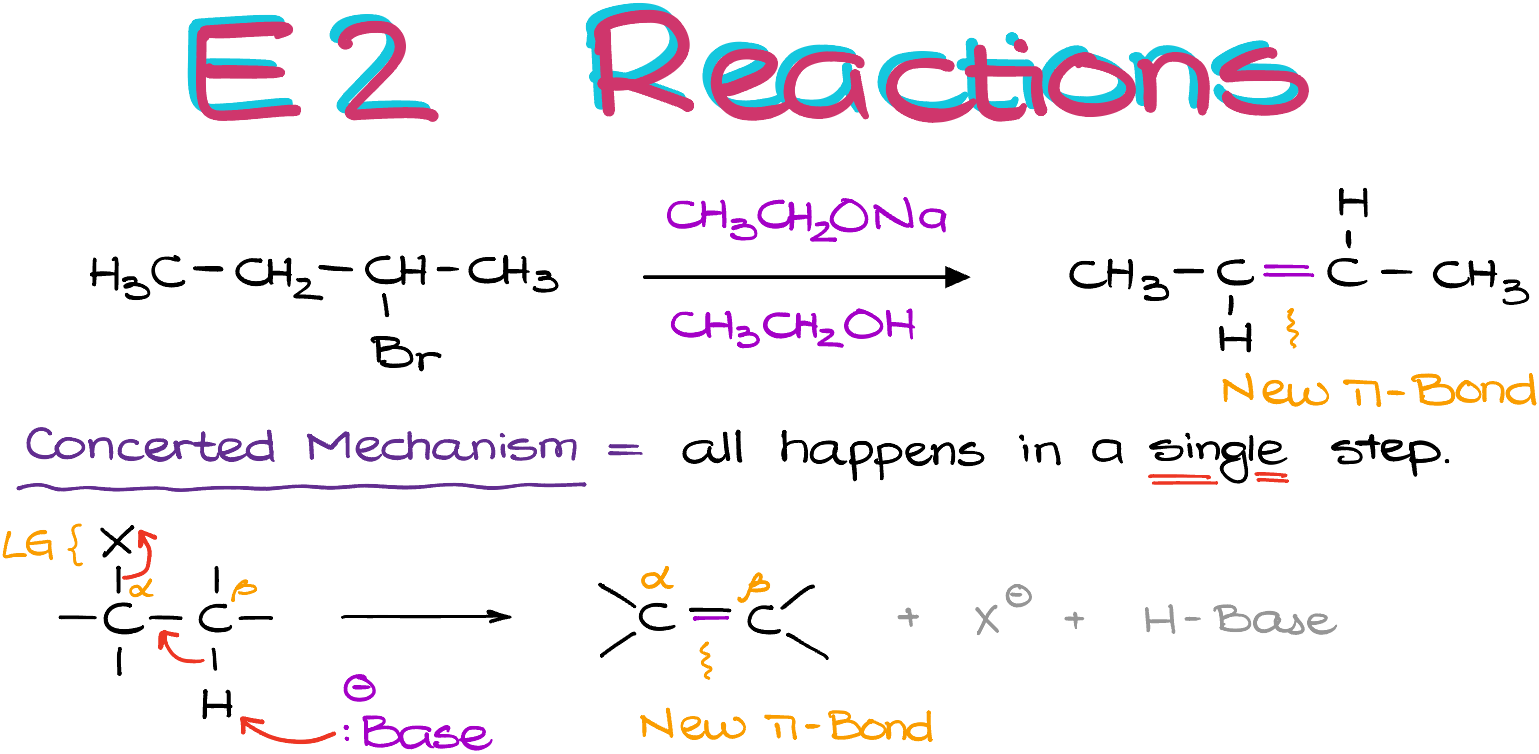
first, the base comes along and “finds” the β-hydrogen that’s on the adjacent atom to our leaving group. I marked my leaving group here as the “X’ group.
Then, as my base is pulling off the proton, this will trigger a cascade of electron density which will displace my leaving group from the molecule. Importantly, this all happens in one step. So, as I’ve mentioned before, we’re not going to form any intermediates.
And as the result, I’m going to see the formation of a new π-bond between the ɑ- and the β-atoms.
Example of an E2 Reaction
So, for instance, let’s look at this reaction:
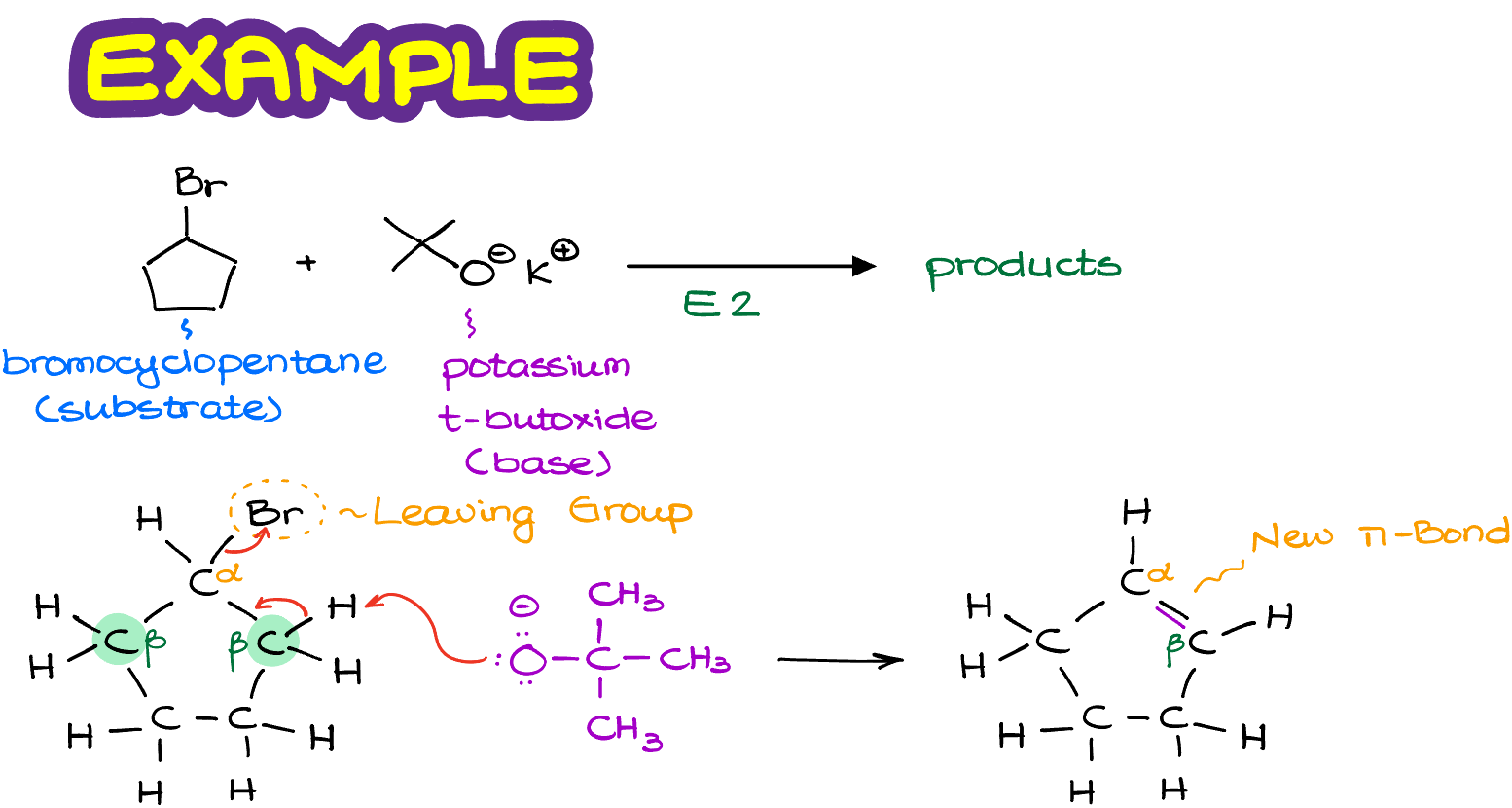
Here I have my substrate, the bromocyclopentane. And my base, potassium tert-butoxide.
And for the clarity’s sake, I’m going to redraw my substrate in a full Lewis structure, so we can see exactly what we’re dealing with and where our atoms are.
The bromine in this molecule is the leaving group, so the carbon on which it is sitting is going to be our ɑ-atom.
Next, I need to find the protons on the β-carbons to see what sort of elimination products I can get in this reaction. And as in this molecule both of our β-positions are identical, I’m only going to focus on one of them as the other will give me exactly the same product.
So, what happens next, is our base comes in and performs the elimination reaction in one step. Notice, that as the potassium here is just a spectator ion, I’m not even showing it in the reaction mechanism as it’s not really doing anything. And just like in the previous example where I used a generic molecule, here we too make a new π-bond between our ɑ- and β-atoms. Easy enough, right?
Examples of Strong Bases Used for E2 Reactions
So, are there any specifics that we need to be mindful about? And yes, of course there are! The firs requirement is that E2 reactions require strong bases.
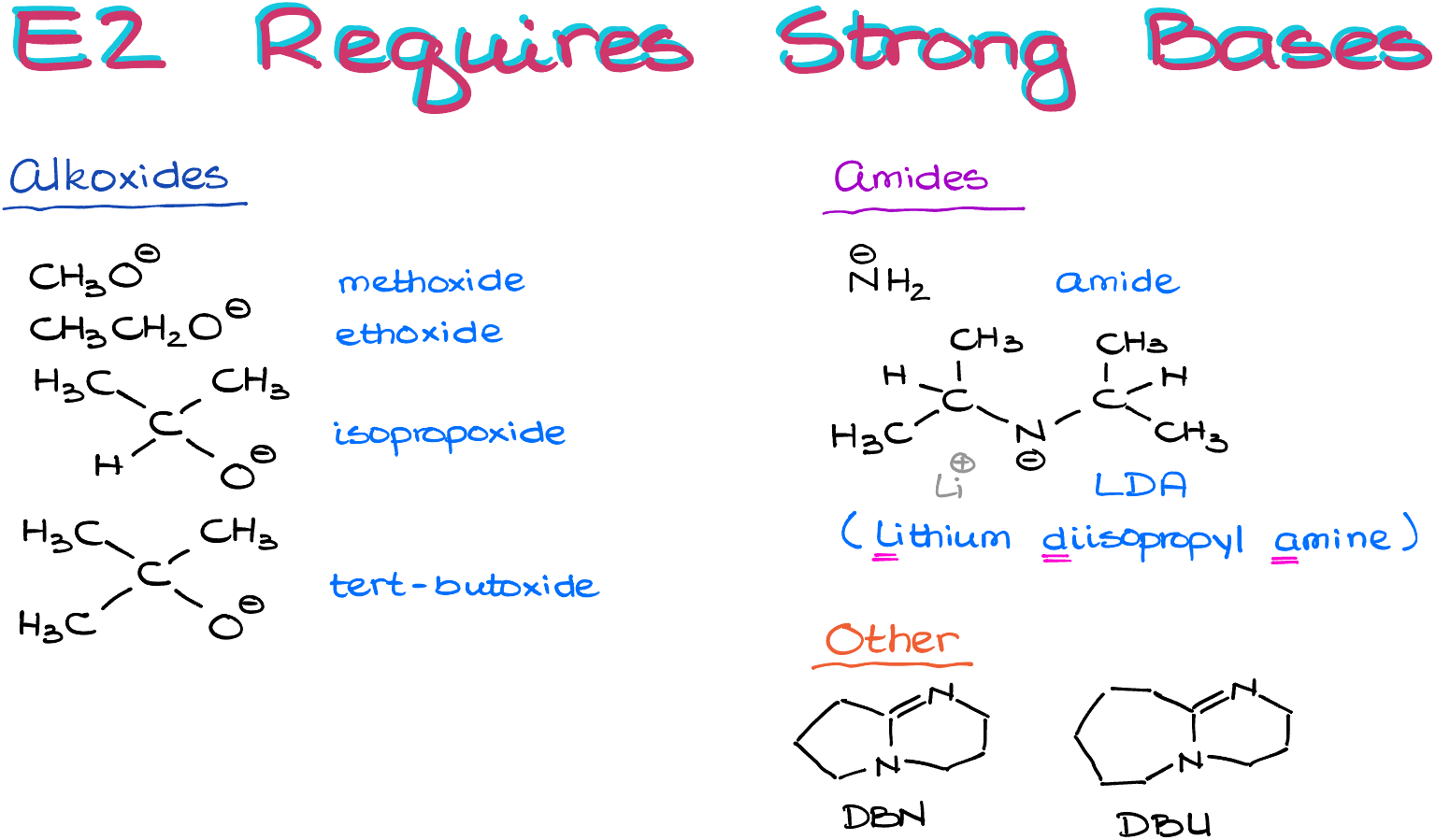
Typical examples of those include various alkoxides like methoxide, ethoxide, t-butoxide, etc. Our alkoxides will typically have some sort of alkali metal as a counter ion, like sodium or potassium. For our purposes, the metals’ nature is pretty much irrelevant, so they are interchangeable. Later on, once we start discussing more advanced concepts, the metals will be more important, but for right now, they are just the spectator ions.
We’ll also see some amides like sodium amide (rarely) and LDA (more often).
Finally, we’ll also occasionally come across exotic bases like DBN or DBU, which stands for diazobicyclononane and diazobicycloundecane, correspondingly.
Make sure you copy those down as it’s really important to know your typical strong bases and be able to recognize them once you see them in the reaction.
Regioselectivity of E2 Reactions Determined by the Base Size
Here’s another important thing about the bases: the size indeed matters. So, we are going to split our bases into a small and a bulky category.

And the reason for why it matters is that the bulky bases are very sensitive to the sterics around the leaving groups.
Let me illustrate how it matters.
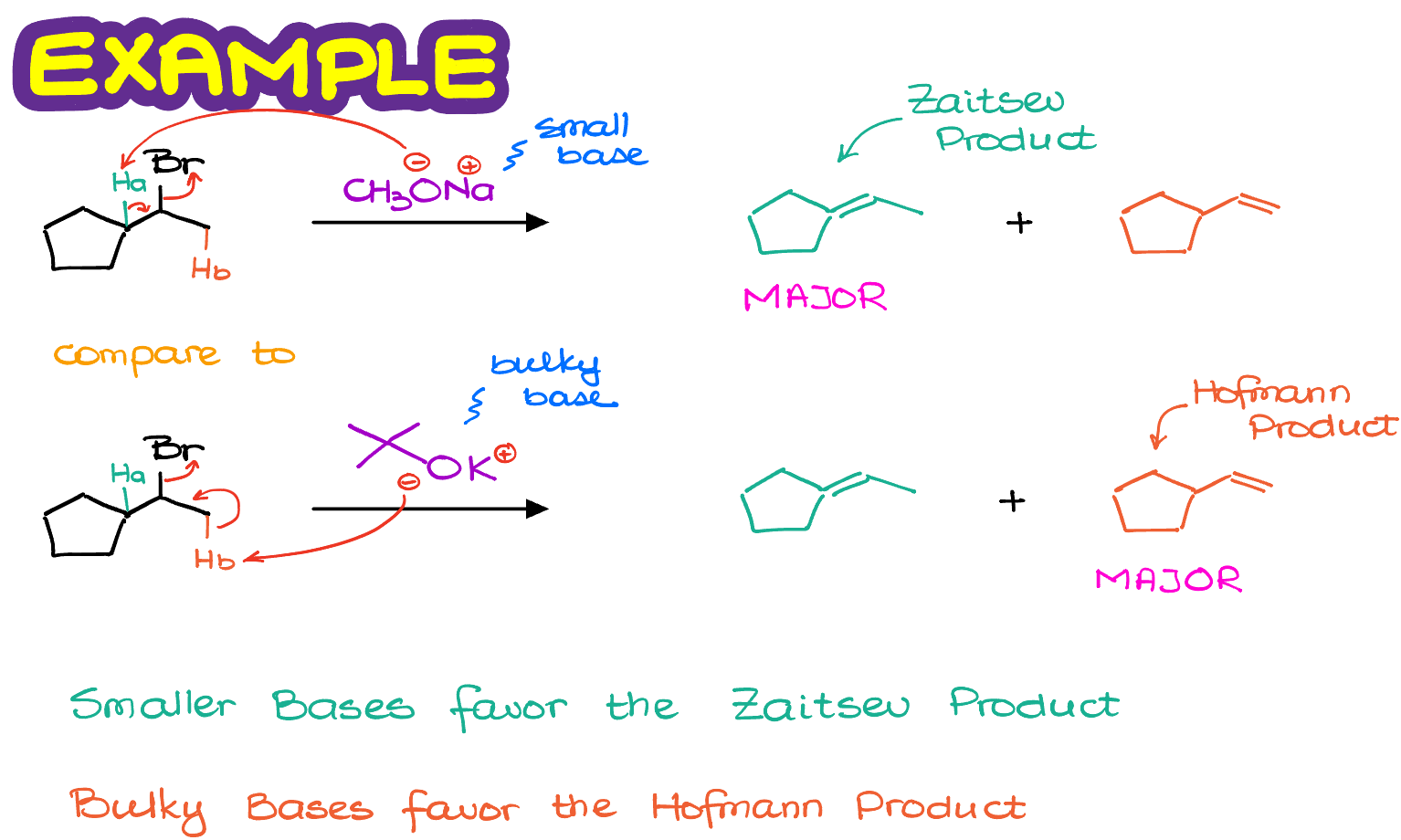
Here I have the same substrate reacting with two different bases. As we have the same substrate, we can potentially make two different alkene products as we have two different β-hydrogens in our substrate. This gives me the following two molecules. The hydrogen “a” is sitting in the middle of the molecule, so it’s pretty sterically hindered. This means, that a bulky base, such as tert-butoxide, will have a lot of troubles trying to reach this proton. So, instead, a bulky base will go after the less sterically hindered proton, such as proton “b” which is sitting on the outskirts of the molecule.
Thus, the more substituted double bond will be the major product if we use a smaller base, and a less substituted bond will result if we use the bulky base.
You can also hear the terms “Zaitsev product” and “Hofmann product” which refer to the two possibilities. The Zaitsev product is an alkene with the most substituted double bond, while the Hofmann product is an alkene with the least substituted double bond out of all possible alkenes that the reaction can produce.
E2 Reaction Energy Diagrams
We can also explain this observation from the perspective of the reaction rates and energy diagrams. In the case of the bulky base, the transition state for the reaction involving proton “a” is going to be significantly higher in energy than a similar transition state for the reaction involving proton “a” in the case of the smaller base. And as the activation energy for the reaction involving proton “a” in the case of the bulky base is very large, the reaction yielding the Zaitsev product with bulky bases will be very slow. Thus, we predominantly go with the formation of the Hofmann product in the case of the bulky bases.
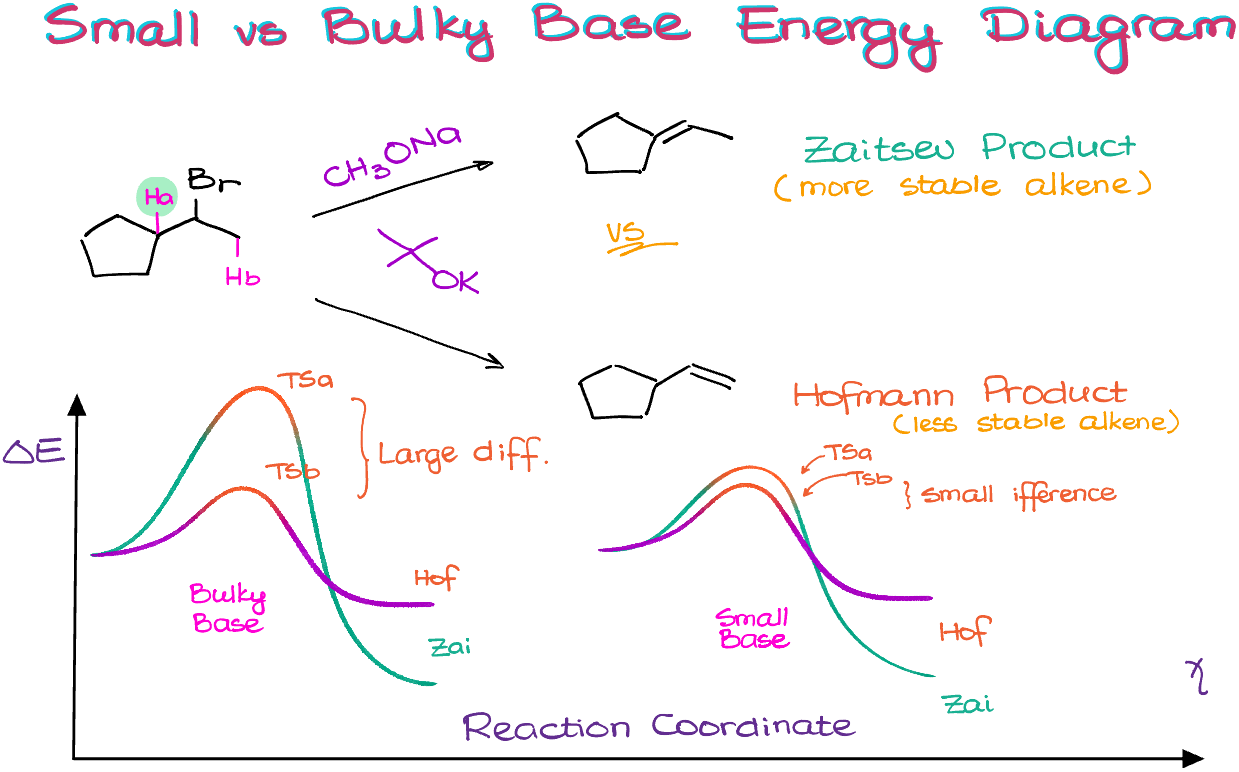
With the smaller bases, however, the activation energy differences for both approaches are very similar. Thus, we are going to go with the pathway leading to the formation of the more stable product, aka, the Zaitsev product.
Stereochemistry of the E2 Reaction
There’s, however, another important requirement that we have for the E2 reactions. And that is the stereochemistry of the transition state.
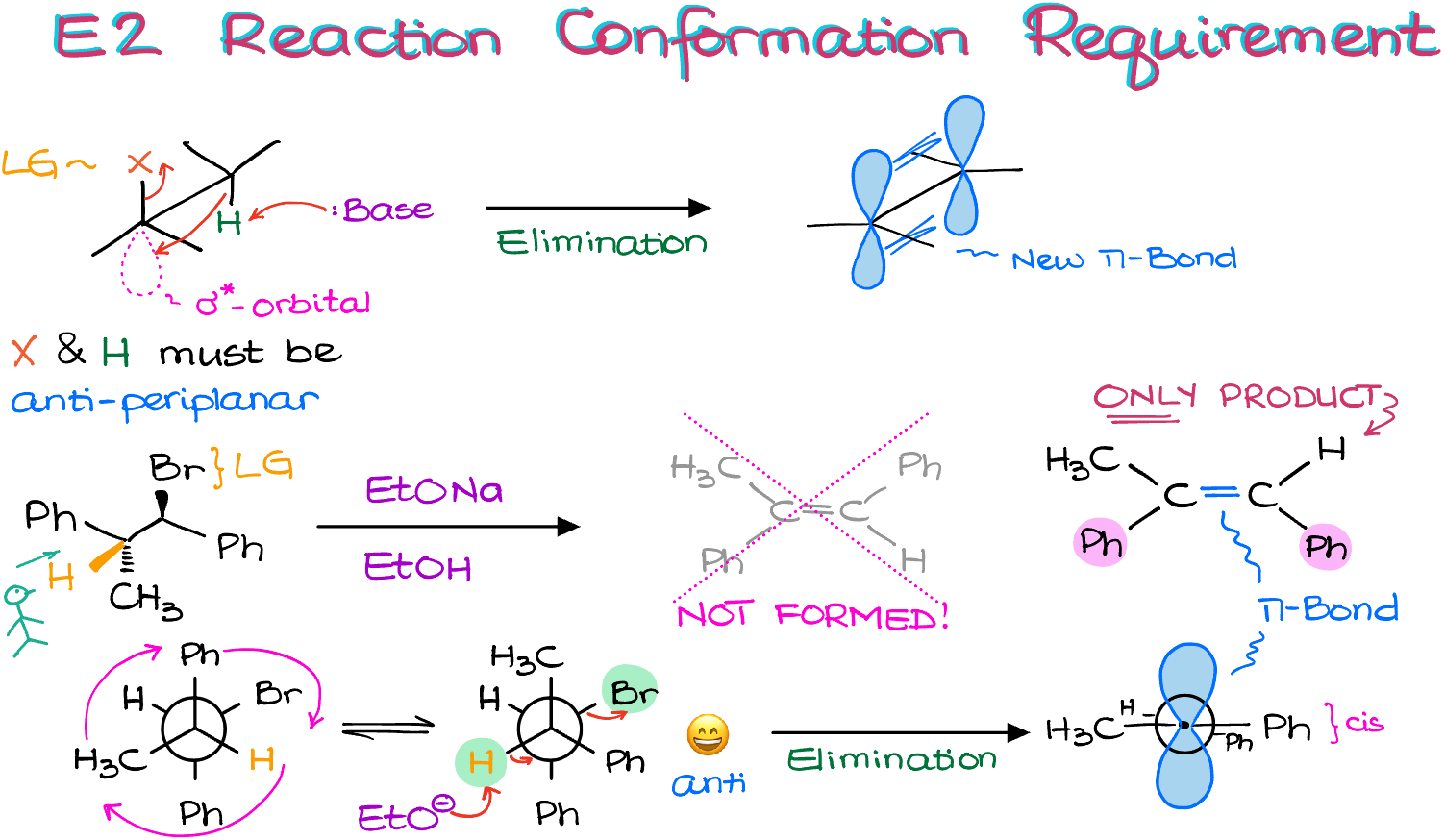
The requirement is that the proton that we’re trying to pull off and the leaving group, X, must be anti-periplanar to each other.
This requirement comes from the molecular orbital interactions in this reaction. In order to push our leaving group out of the molecule, we need to supply the electrons from the C-H bond onto the C-X σ* anti-bonding orbital. And that is only possible if the C-H bond is coplanar with the said σ* orbital.
This cascade of electron density will then result in the formation of a new π-bond. Thus, if the atoms are not anti-periplanar, you’ll need to be able to rotate the carbons around the single bond to make them. Otherwise, the elimination reaction is going to be impossible. Well, impossible via the E2 mechanism, that is. We could possibly still have eliminations occurring via E1 or E1cB mechanism. But the anti-periplanar orientation of the H and the leaving group for the E2 reaction is pretty strict, so we cannot skip it.
For instance, let’s look at this example above.
The substrate has a good leaving group in the form of the bromine atom. When it comes to the protons on the β-carbons, well, we only have one. And those two groups are not anti-periplanar! Let me illustrate it by drawing a Newman projection looking from the left.
The Br atom and the H atom that I’d like gone are actually gauche to each other instead of being anti. Which is a problem because we know that they need to be anti for the reaction to occur. So, what are we gonna do?
We’ll rotate one of our atoms around! NOW, they are indeed anti-periplanar to each other. Which means that the reaction is going to be possible.
Here’s the Newman projection for the product. By comparing what we had before the reaction and how the process forces the atoms in the resulting product, we can see that we are going to end up with a very specific stereoconfiguration.
The important observation here is that the phenyl groups are going to end up cis to each other. While the other stereoisomer would be more stable because it would have the bulky groups further from each other it’s NOT going to be formed and the cis molecule will be the ONLY E2 product in this reaction.
So, if you don’t have a hydrogen that’s opposite (aka, anti) from your leaving group, make sure you do the Newman projection analysis. I can also guarantee that this IS going to be on your exam. This is such a classic aspect of the E2 reaction that every single instructor I’ve ever seen tests for it.
Stereochemistry of E2 Reactions in Cyclohexanes
This, naturally, also applies to the cyclic substrates. For instance, let’s look at this example here:
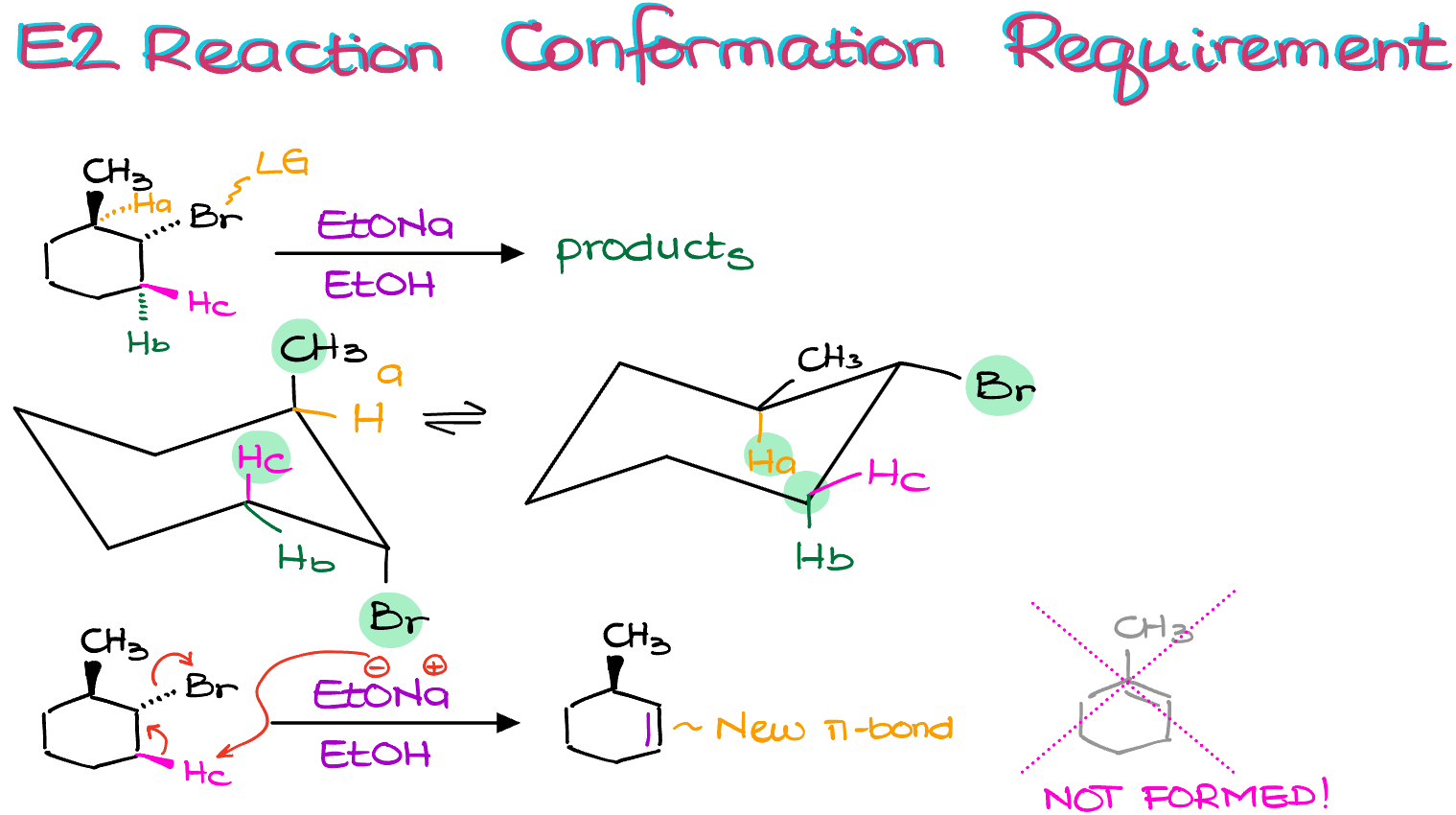
By doing the preliminary analysis, we can see that we have a few protons in the β-position to our leaving group. Let’s call them Ha, Hb, and Hc.
As building a Newman projection for a cyclic compound is a little difficult (although quite doable, so you should probably challenge yourself and draw one!), we’re going to make a chair conformation instead.
In the left chair conformation, my bromine is anti to the Hc and the methyl group. The methyl group is, of course, useless for us, but Hc works for our purposes. In the right chair conformation, however, the only atoms that are anti to the bromine are the carbons of the ring itself. So, no protons can be used in this conformation.
Notice, I don’t care which conformation is more stable. I ONLY care IF I have an “anti” proton to my leaving group. The only time when I’ll be considering the conformation stability is when I’ll be discussing the reaction rates. Till then, the conformation stability is irrelevant for me. Like in this case, even though the right conformation is more stable, reaction is impossible from that conformation. So, the fact that this conformation is more stable is completely irrelevant for our purposes.
Thus, since we now know that only Hc is useful for us, we can proceed with the reaction.
As you can see, the “anti” requirement is a pretty big deal for the E2 reactions. So, always make sure you’re checking for it before you propose your product and don’t just blindly go for the “most stable product” without doing the appropriate analysis no matter how tempting it might be.
