E1 Reactions
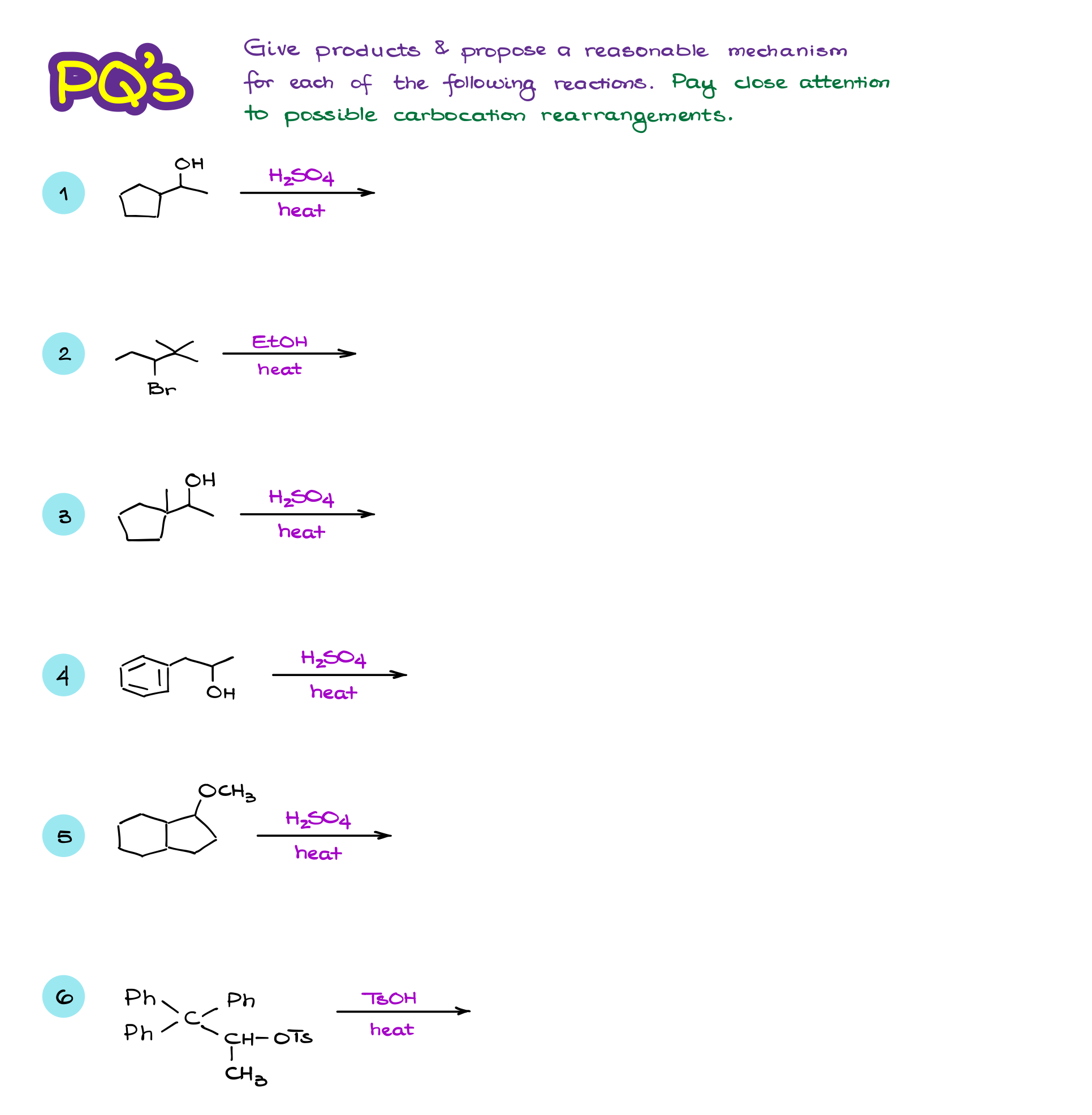
So, here we have a seemingly straightforward reaction.
Going through the mechanism here, we see a typical leaving group dissociation here, which is favored because we are working in polar protic conditions and have the leaving group at a 3° atom. We then have a nucleophilic attack from the acetate, which is a conjugate base of our solvent. So, we expect the final product to be this molecule. Right?
…but turns out we also get an alkene in this reaction. But, it gotta be a minor product, right?
…not necessarily either. It really depends on the conditions of our reaction!
So first of all, let’s see how the alkene product forms in this reaction.
The acetate anion that we have acting as a nucleophile in this reaction, can also act as a base. The thing is, carbocations are wickedly acidic. And while we don’t really have an actual pKa value for them, it’s been estimated to be somewhere around -12 to -25. Which is, for the lack of a better word, ridiculously acidic. So, even a weak base like an acetate ion can easily deprotonate them making a double bond.
Temperature Effect on E1 Reaction Favorability
The question is though: how can we control our reaction and get one or the other to be the major product?
As E1 reactions are unimolecular eliminations and follow the same rate law as the SN1 reactions, the amount of our base or a nucleophile is irrelevant. So, what does matter then?
Well, it turns out, the temperature is one of the key aspects here.
If we accept that at the micro level all our reactions are reversible, that is before we form any gaseous products that may escape, or any precipitates that may crystalize, then we can use the laws of thermodynamics to look at how different processes are favored.
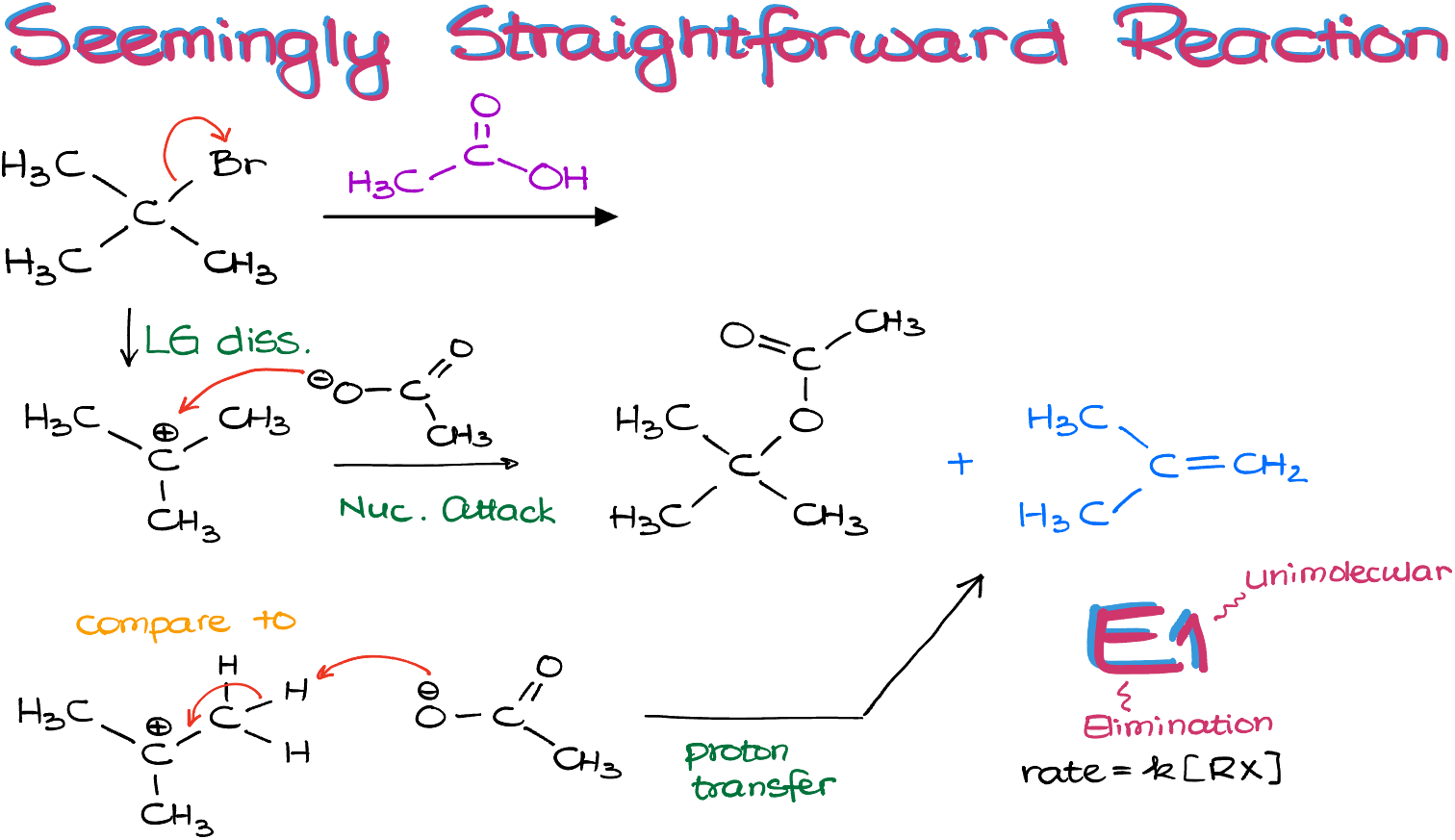
Let’s start by writing out both of our possible reactions as regular balanced reactions like we used to do in general chemistry.
We can see, that in the case of the elimination reaction, E1, the acetic acid was not actually consumed at all, so I can cross it out from my equation.
Next, we can also see that the first reaction gives us 2 moles of products and consumes 2 moles of reagents. While the second reaction only consumes 1 mole of reagents, while producing 2 moles of products.
This means that the change in entropy of the first reactions (ΔS) is negligible. While the change in entropy for the second reaction, the elimination, is some positive value.
Why is this important to notice? Well, going back to what we’ve learned in the thermodynamics module on our general chemistry course (I know you’ve been really trying to purge that from your memory as a bad dream, but we unfortunately need it all back), if the reaction has a negative free Gibbs energy (ΔG) it’s going to be spontaneous. And what is ΔG?
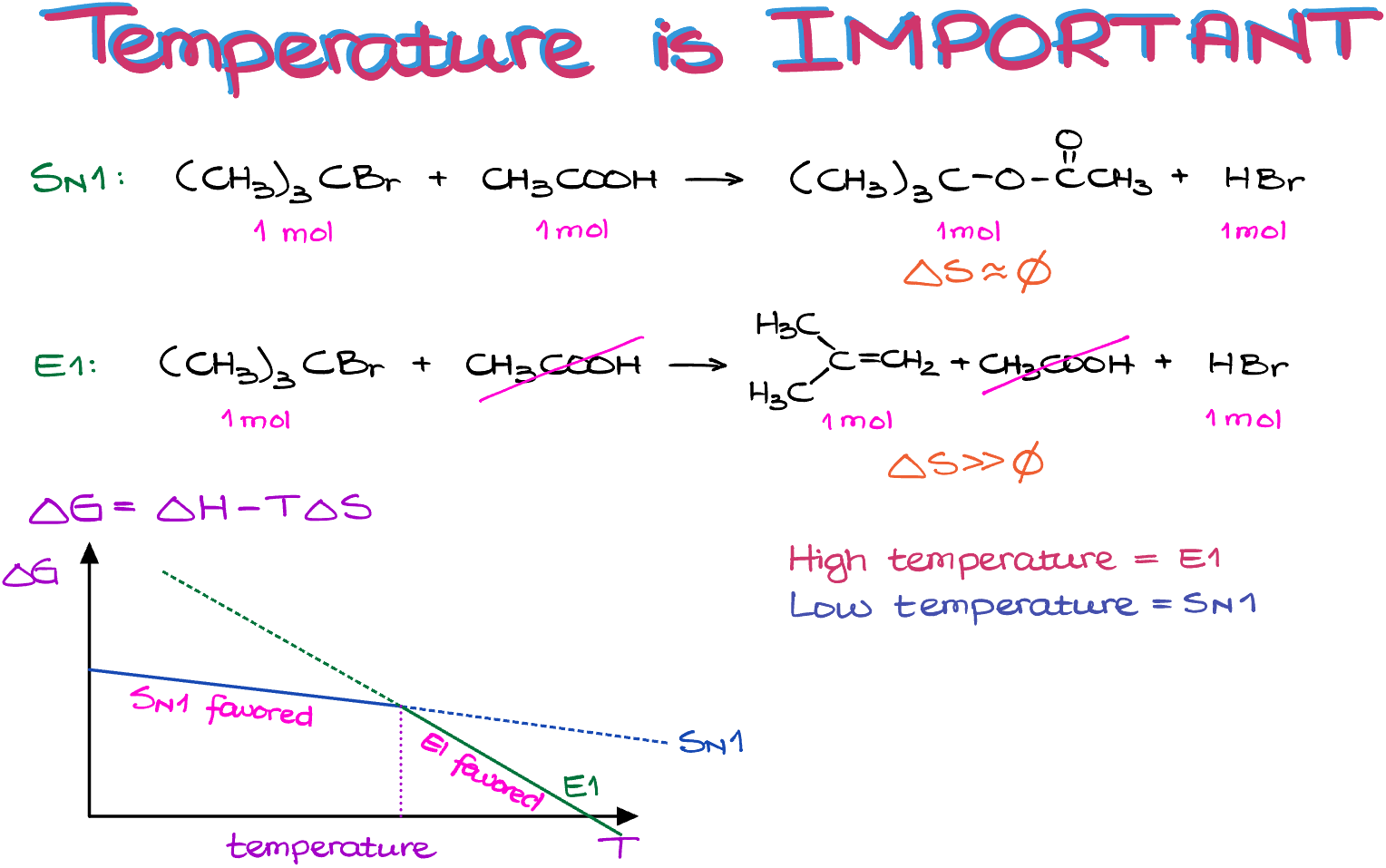
ΔG = ΔH – TΔS.
So, as the change in entropy is negligible for the substitution reactions, they depend on the temperature very insignificantly. However, the elimination reactions, with their large positive change in entropy, are very sensitive to temperature and become more favorable with higher temperature.
So, if I plot the ΔG as a function of temperature for both processes, we can see that up until certain temperature, the substitution is more favorable. But as soon as we cross that threshold, the elimination reaction becomes more favorable.
In a nutshell, you can remember, that if you’re on a fence about the reaction and can’t decide if it should be an SN1 or an E1 reaction, check the temperature. Higher temperatures will favor elimination, while lower temperatures—substitution.
Example of an E1 Reaction
For instance, let’s look at this reaction.
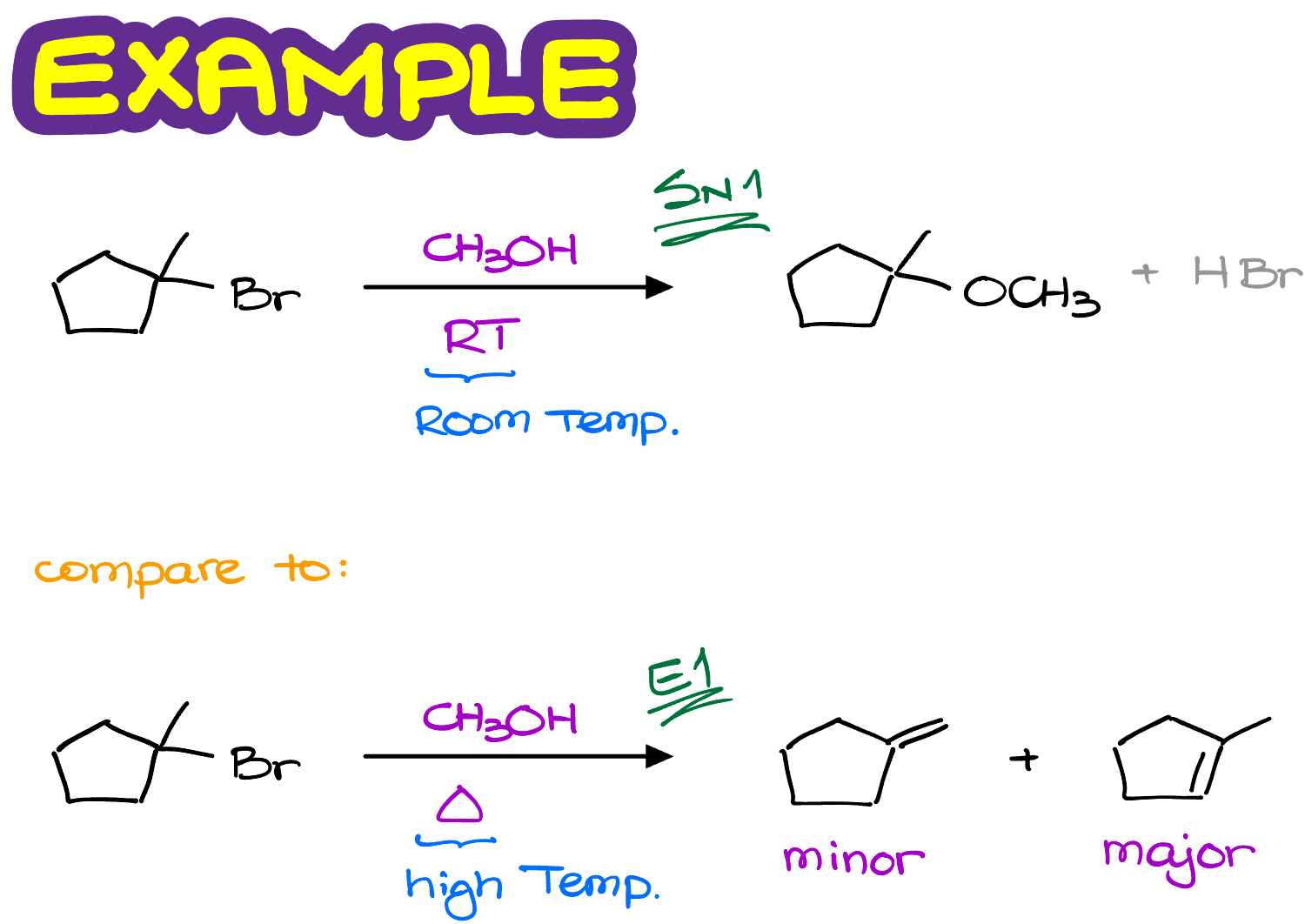
We have the same substrate molecule reacting with the same methanol, but the temperature is different.
In the first case we are going to see a typical SN1 reaction in which the -OCH3 replaces the -Br in our molecule.
While in the second case, we are going to see the E1 reaction making some alkenes.
Notice, that in this reaction we are actually making two different alkenes and one of them is the major product. Which brings me to the topic of the regioselectivity of the E1 reactions.
Regioselectivity of the E1 Reactions
Let’s work through the mechanism of this reaction:
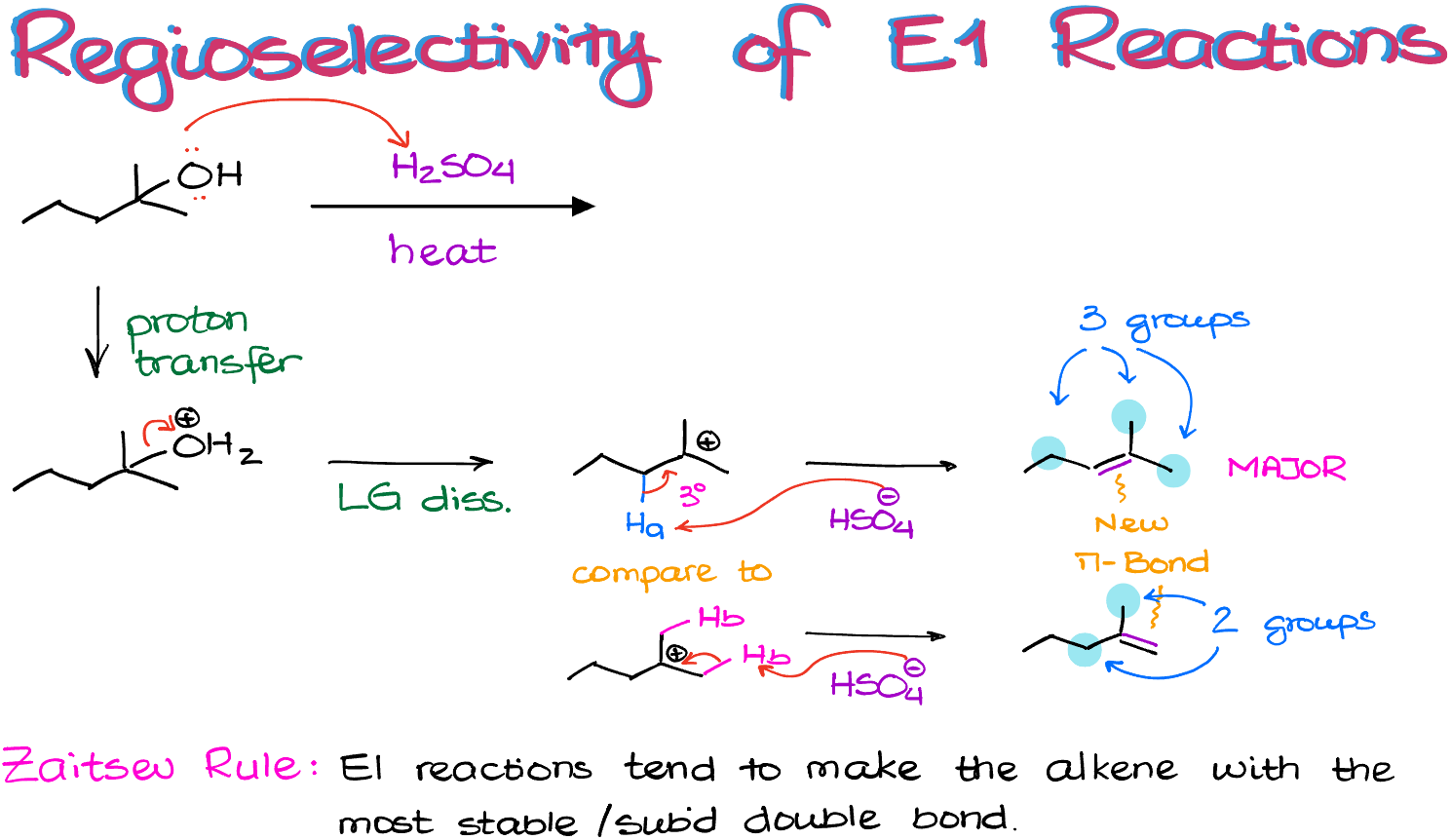
The first step in this reaction is going to be a proton transfer giving us a good leaving group.
Next, upon the leaving group dissociation, we are going to make a 3° carbocation. As this carbocation is already tertiary and there are no other opportunities to make it even more stable, we are not going to expect to see any carbocation rearrangements in this molecule.
Thus, the next thing that we are going to do is to use some sort of a base to pull a proton off and make an alkene.
Here, we have two different types of hydrogens that we can pull off to make a double bond. As those hydrogens are sitting on the adjacent to a carbocation atom, we are going to refer to them as β-hydrogens. So, I have a β-hydrogen “a” (strictly speaking, there are two of those, but I’m just showing one here), and we have another β-hydrogen “b” (and we have a grand total of six of those, three per each methyl group).
If I use the conjugate base of the sulfuric acid (or, alternatively I can use H2O here) to deprotonate my molecule at the position “a”, I’m going to end up with a new double bond in the middle of the molecule.
If I do the same trick with one of my other hydrogens—hydrogen “b”—then I’m going to end up with a double bond on the edge of the molecule.
And here’s something important: these two double bonds are not the same!
The one on top is attached to 3 non-hydrogen groups.
While the one on the bottom is only attached to 2 non-hydrogen groups. This makes the top double bond more substituted. And generally, the more substituents we have on a double bond, the more stable it’s going to be.
Typically, E1 reactions favor the formation of the more stable double bond! This is known as a Zaitsev rule named after Russian chemist Alexander Zaitsev (Александр Зайцев) from the Kazan University who originally discovered it.
Thus, the top product is what we would expect as the major product in this reaction. All other possible products will still form, but the major one will always be the one with the most stable double bond, which is typically the most substituted one. And yes, there are cases when it is not true, but I’ll talk about those in a different tutorial.
Practice Questions
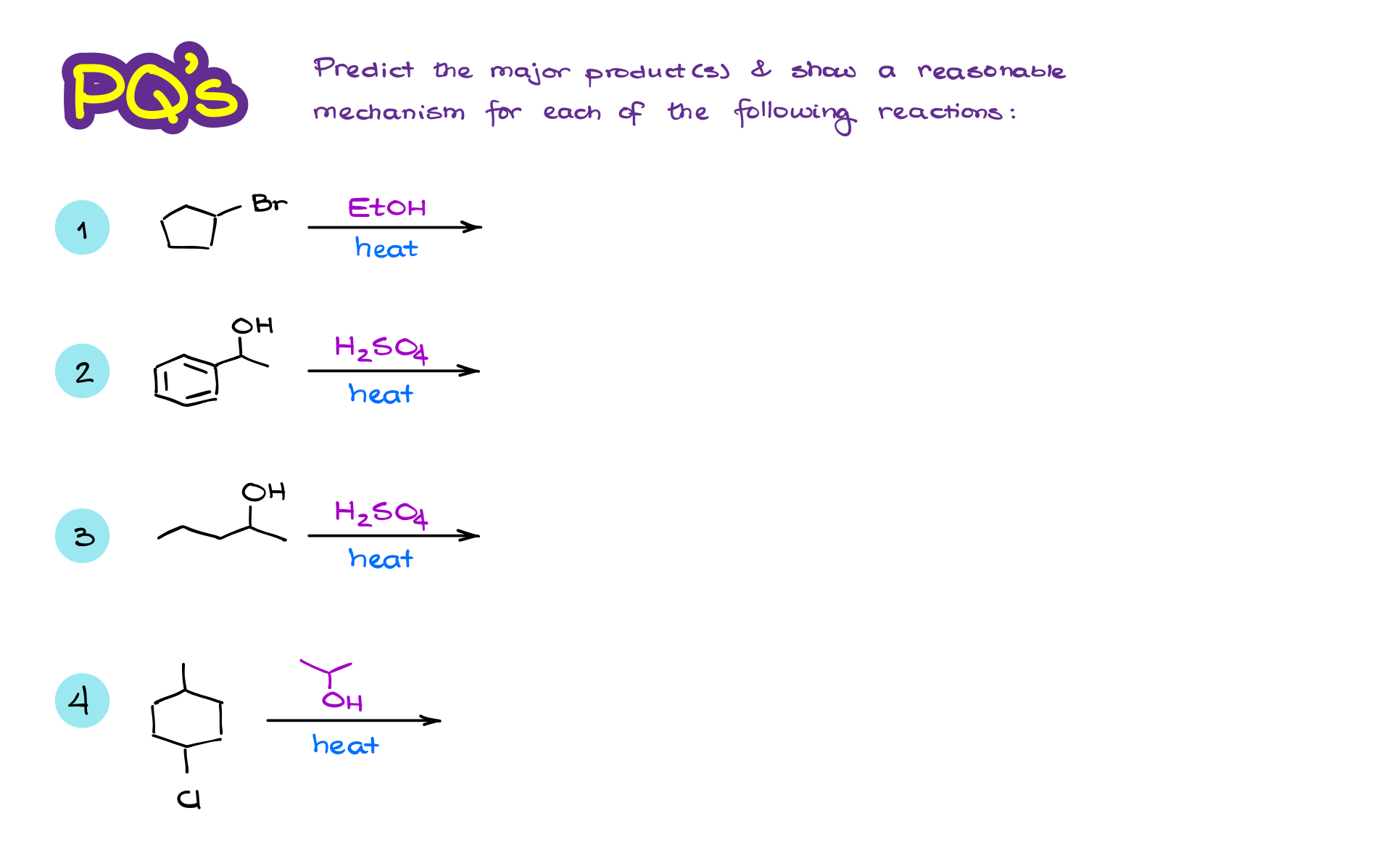
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!
Carbocation Rearrangements in E1 Reactions
Now, E1 reactions do form carbocations as the intermediate. What does that mean? Trouble! If we see a carbocation, we always need to double check our rection for possible carbocation rearrangements! I have a tutorial dedicated to the carbocation rearrangements in SN1 reactions. And all the principles of the carbocation stability and the driving force for the carbocation rearrangements apply here as well. So, if you haven’t seen that video yet, go and check it out now before continuing with this one.
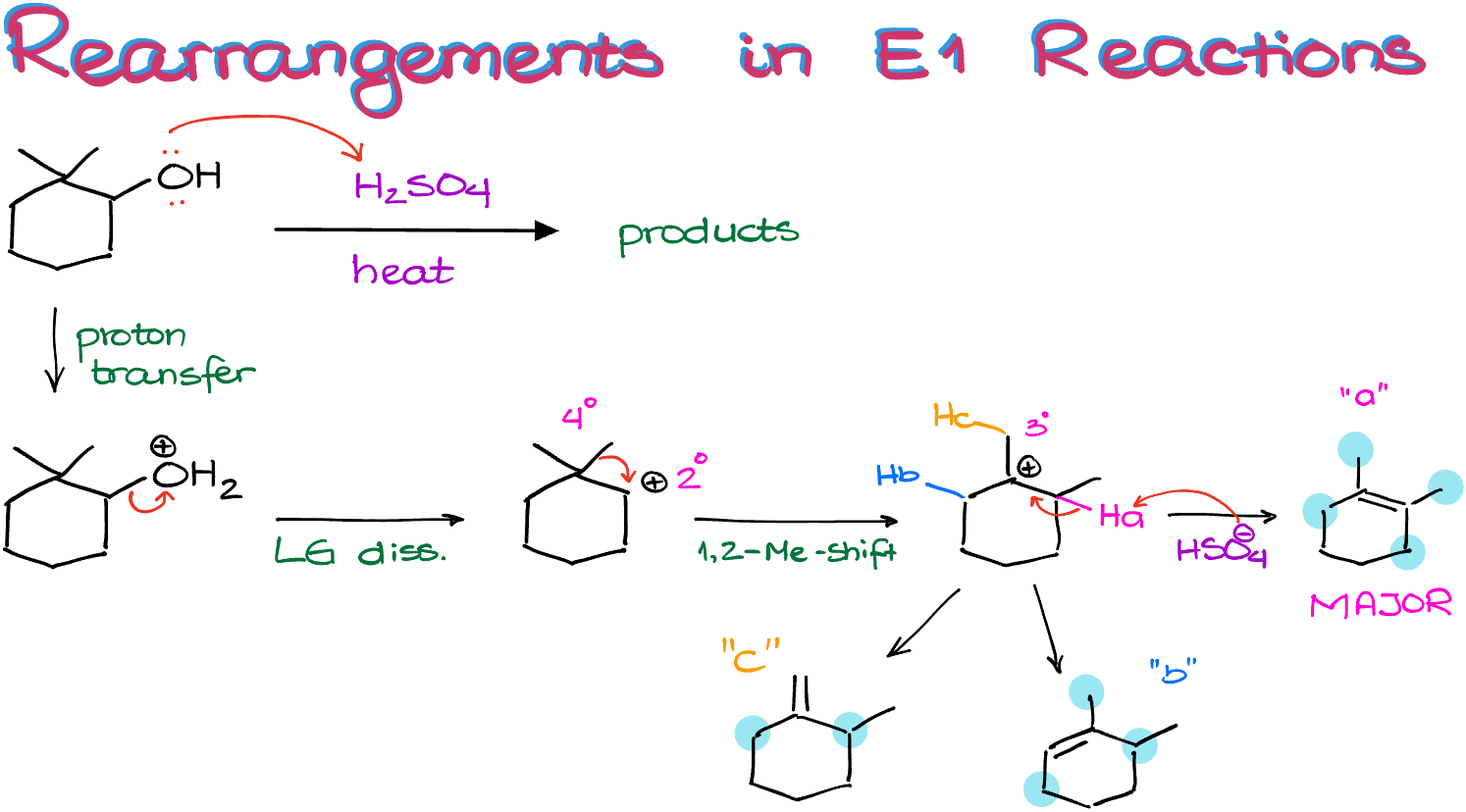
Alright, let’s look at this example over here. This is a typical E1 reaction where an alcohol is treated with a strong non-nucleophilic acid to cause elimination of water. Because we’re losing water here, reactions like this one are often called “dehydration” reactions.
So, the first couple of steps in this reaction will be exactly what we’d expect: the proton transfer followed by the leaving group dissociation. This is going to give us a 2° carbocation right next to a 4° carbon atom. This means that we have a good chance of a carbocation rearrangement.
The methyl shift in this molecule will yield a 3° carbocation, which is more stable than the 2° one.
From this point on, we have a few options for the elimination products.
We have hydrogen “a”, hydrogen “b”, and hydrogen “c” as the potential candidates for the elimination.
If I eliminate the hydrogen “a” I’m going to end up with the double bond with four substituents.
If I do the same to the hydrogen “b” I’m going to end up with a double bond which is only connected to three other non-hydrogen substituents.
And finally, if I eliminate hydrogen “c” my double bond will only have two non-hydrogen substituents on it.
Thus, if we apply the same logic from our previous example, we can conclude that the very first elimination product I came up with, product “a,” is our major product in this reaction.
So, when you’re dealing with the E1 reaction, always check for the carbocation rearrangements, and make the most substituted double bond as your major product.
E1 with Rearrangements Practice Questions