Drawing Pi Orbitals in 3D
In organic chemistry, visualizing molecules in 3D is crucial for understanding their structure and behavior, especially when dealing with pi bonds and conjugation. In this tutorial, we’ll explore how to draw 3D representations of molecules like ethylene (C₂H₄) and butadiene, focusing on the challenges and solutions for showing pi orbitals and ensuring clarity in your drawings.
The Classic 3D Representation: Ethylene (C₂H₄)
Let’s start with a simple molecule like ethylene. The traditional way to draw ethylene in 3D involves representing its atoms and bonds with dashes and wedges to indicate their orientation in space.
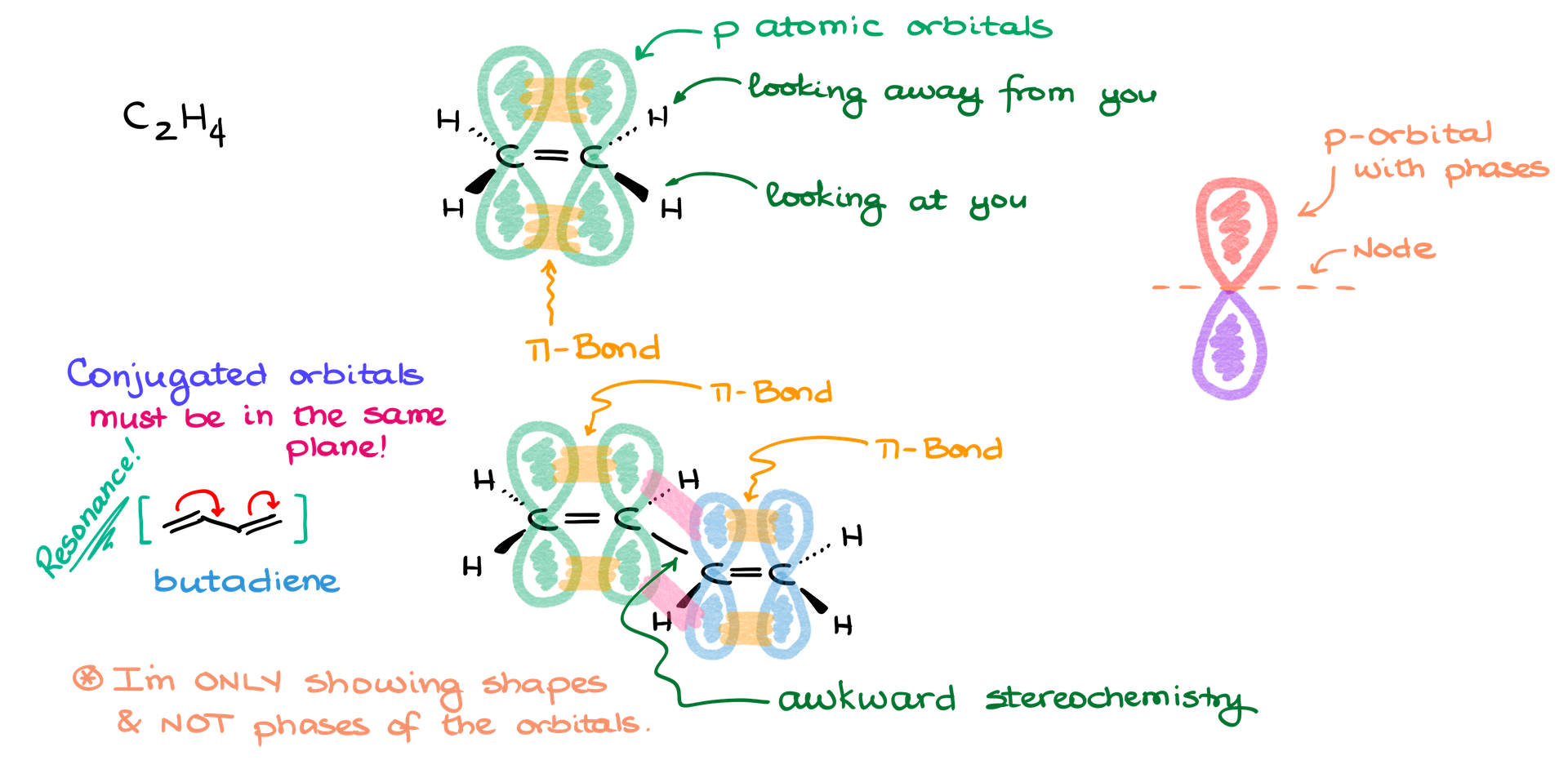
Steps:
- Draw the carbon skeleton: Place two carbon atoms (C) connected by a double bond.
- Add hydrogen atoms: Ethylene has four hydrogens—two are connected to each carbon. Use wedges and dashes to indicate spatial orientation. Wedges represent bonds coming out of the plane toward you, while dashes represent bonds going behind the plane.
- Show pi bonds: The p orbitals that form the pi bond overlap above and below the plane of the carbon atoms. Draw two “lobes” for each carbon’s p orbital, overlapping to form the pi bond.
This is the method you’ve likely seen in textbooks. However, as molecules become more complex, this approach can introduce awkwardness and confusion when it comes to visualizing stereochemistry and bond angles.
Drawing a Conjugated Molecule: Butadiene
Butadiene (C₄H₆) introduces a new challenge: conjugated pi bonds. Conjugation means that the double bonds are not isolated but interact with each other through resonance, creating one large molecular orbital.
Steps:
- Draw the carbon skeleton: Butadiene consists of four carbon atoms, with alternating single and double bonds.
- Add hydrogens: Attach hydrogens to the carbons using wedges and dashes as needed to represent their orientation.
- Draw pi orbitals: For each double bond, represent the p orbitals of the carbon atoms. Since these bonds are conjugated, make sure the p orbitals on all carbons are drawn in the same plane. You can show the overlap between the orbitals on adjacent carbons to represent the delocalized pi system.
- Key Concept: The double bonds in butadiene participate in resonance, so the orbitals forming the pi bonds must be coplanar. When drawing conjugated systems, ensure that the pi bonds interact by showing orbital overlap across the molecule.
Avoiding Stereochemical Awkwardness: Top-Down View
As molecules become more complex, drawing them with dashes and wedges can lead to confusion. For example, butadiene’s middle bond can look awkward in 3D, with wedges and dashes creating unclear representations. To avoid this, consider using a top-down view.
What is the Top-Down View?
In this method, the molecule lies flat in the plane of the paper, while the p orbitals, which form the pi bonds, are shown perpendicular to the plane, piercing through the paper.
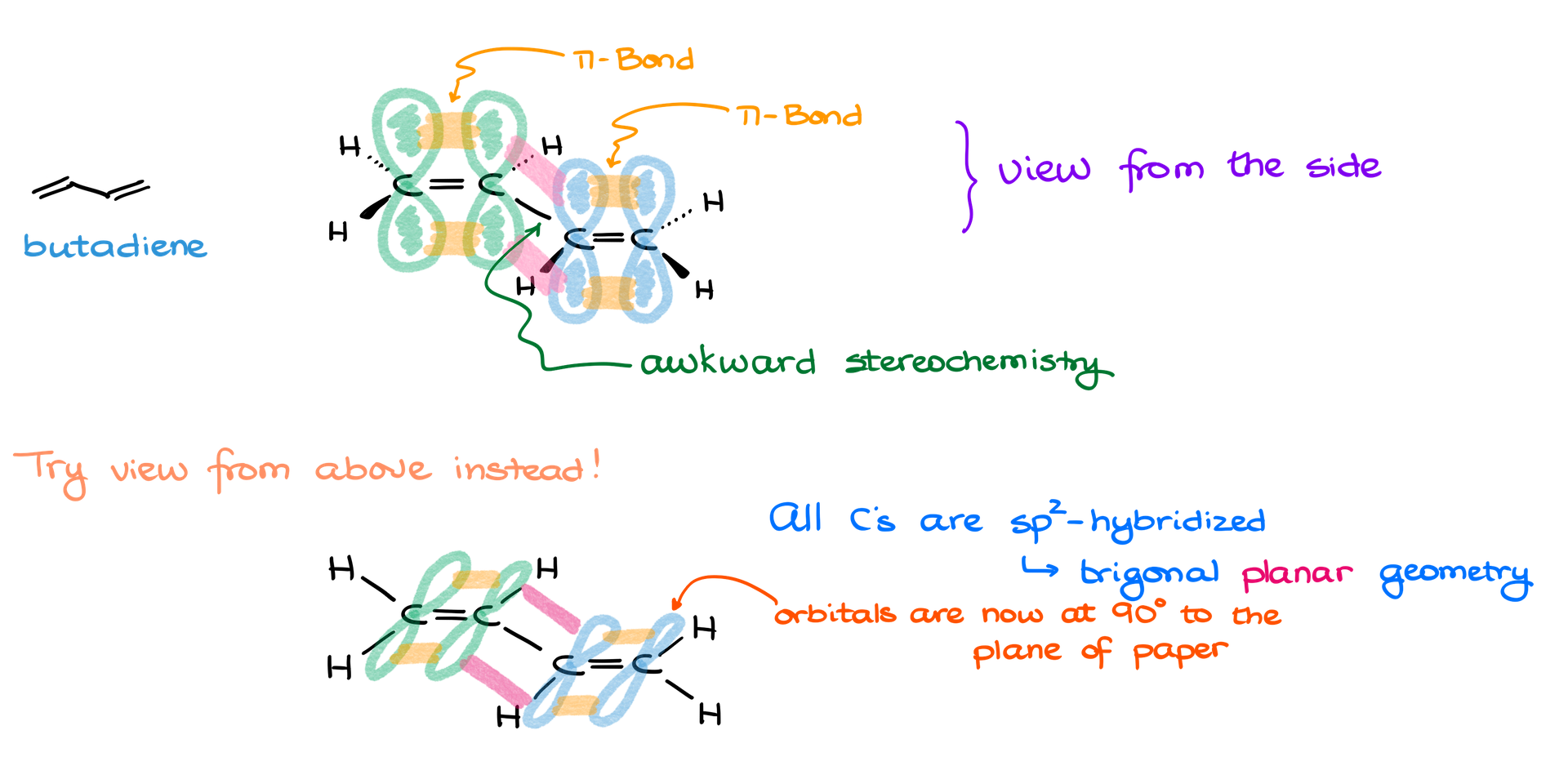
Steps:
- Draw the flat carbon skeleton: Butadiene’s carbons are sp² hybridized, which gives the molecule a flat, trigonal planar geometry. Draw the skeleton without worrying about wedges or dashes.
- Add the pi orbitals: Draw the p orbitals above and below the plane of the paper, perpendicular to the carbon atoms. Show the overlap between the orbitals to represent the pi bonds.
- Show conjugation: Make sure the p orbitals on adjacent carbons are drawn interacting, showing the resonance delocalization in a single conjugated system.
- Why It’s Useful: This approach removes the need for wedges and dashes, simplifying the drawing while ensuring that orbital interactions are clear. It may look unusual at first, but it eliminates ambiguity in stereochemistry and orbital placement.
Example: Drawing Acetamide in 3D
Let’s apply the top-down approach to a more complex molecule like acetamide (CH₃CONH₂), which has both pi bonds and electron pairs that participate in resonance.
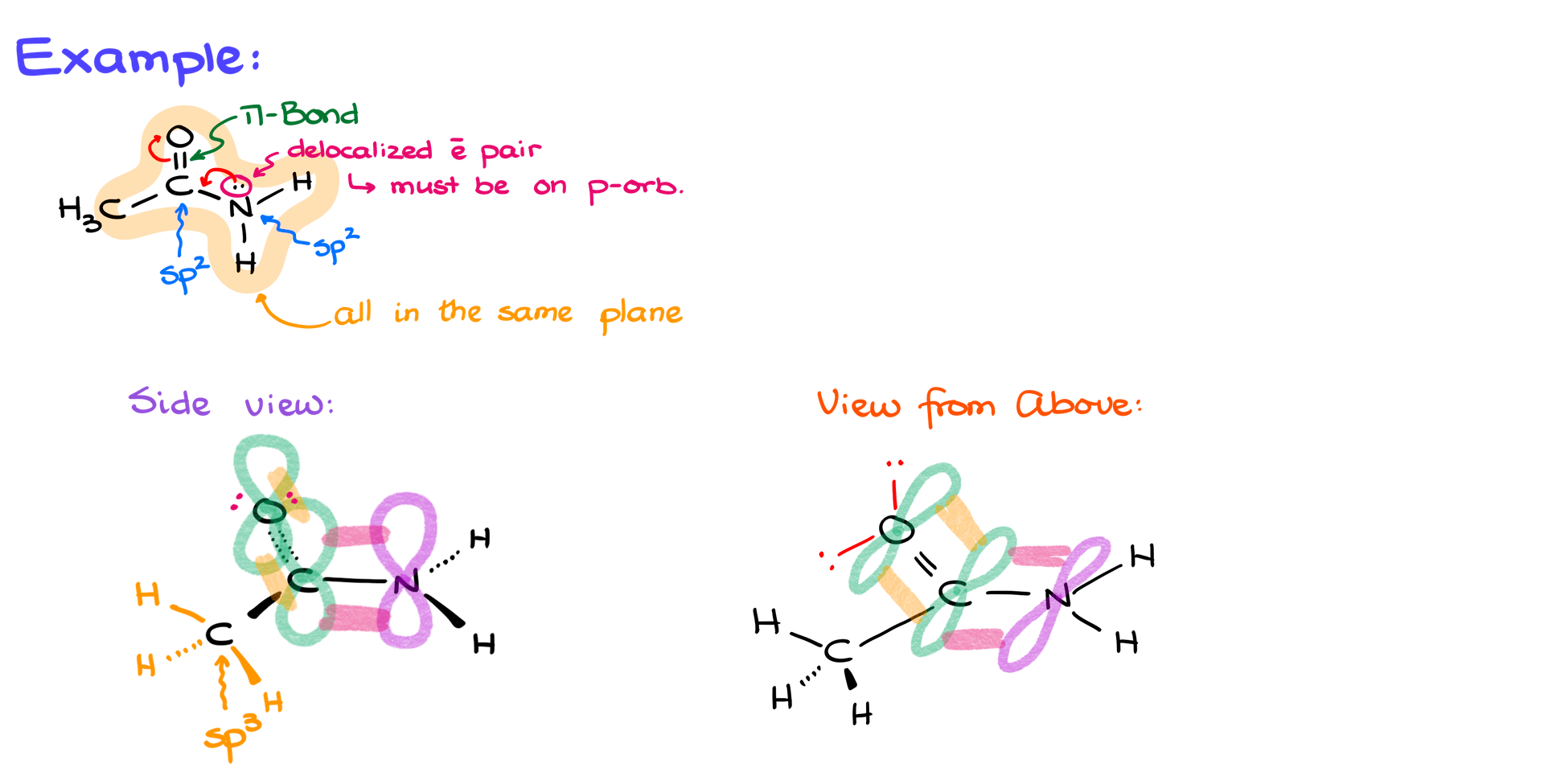
Steps:
- Identify the key features: Acetamide has a pi bond between carbon and oxygen, as well as a delocalized electron pair on nitrogen that participates in resonance.
- Determine hybridization: The nitrogen and carbon involved in resonance are sp² hybridized, meaning the molecule is mostly planar.
- Draw the molecule flat: Draw the carbon, oxygen, and nitrogen atoms in the same plane, as well as the hydrogen atoms attached to nitrogen.
- Add p orbitals: Show the p orbitals on the carbon and oxygen forming the pi bond. Also, show the p orbital on nitrogen where the electron pair resides.
- Represent resonance: Indicate the conjugation between the carbon, nitrogen, and oxygen by showing the interaction of their p orbitals, representing resonance delocalization.
- Alternative Side View: If you choose to draw acetamide from the side view, you’ll need to show wedges and dashes for the hydrogens and other atoms. However, this can result in awkward representations, especially when multiple stereochemical elements need to be clearly shown.
Key Takeaways and Final Tips
Both the side view and top-down view approaches have their advantages. The side view is more traditional but can be difficult to use with complex molecules, while the top-down view simplifies spatial orientation at the cost of looking slightly unusual.
- Practice Both Methods: Try both approaches to see which one works best for you and the molecule you’re trying to represent.
- Avoid Ambiguity: The most important rule is to ensure your drawing is unambiguous. If the 3D orientation of bonds, orbitals, or electron pairs is unclear, it could lead to mistakes in analysis or cost you points on exams.
- Visual Clarity: Whether you use wedges and dashes or a top-down view, make sure that bond angles, orbital interactions, and stereochemistry are clearly represented.
Drawing molecules in 3D can be tricky, but with practice and the right approach, you can master it. Whether you’re using the traditional side-view method or adopting the top-down view, the goal is to create clear and accurate representations of molecular structures and their interactions. Keep practicing both methods, and remember, the key is clarity and understanding the spatial arrangement of atoms and orbitals in 3D space.

Why is the Nitrogen in the last example SP2 hybridized, not SP3 hybridized? It has 4 “things” that it’s attached to? Does the delocalized electron pair affect this? Thanks!
Yes. It participates in resonance, so the electron pair has to be on the p-orbital. So, it has to be sp2-hybridized. You can review hybridization and resonance where I talk about it:
Here and here.