Drawing Bond-Line (Skeletal) Structures
Bond-Line Structures: A Detailed Guide
Bond-Line structures, also known as Skeletal structures, are a fundamental part of organic chemistry. They offer a concise and efficient way to represent organic molecules, contrasting with the detailed and often tedious nature of Lewis structures.
Understanding Bond-Line Structures
Drawing organic molecules using Lewis structures involves showing all atoms and bonds. For larger molecules, this approach becomes increasingly cumbersome. To circumvent this, chemists employ Bond-Line structures.
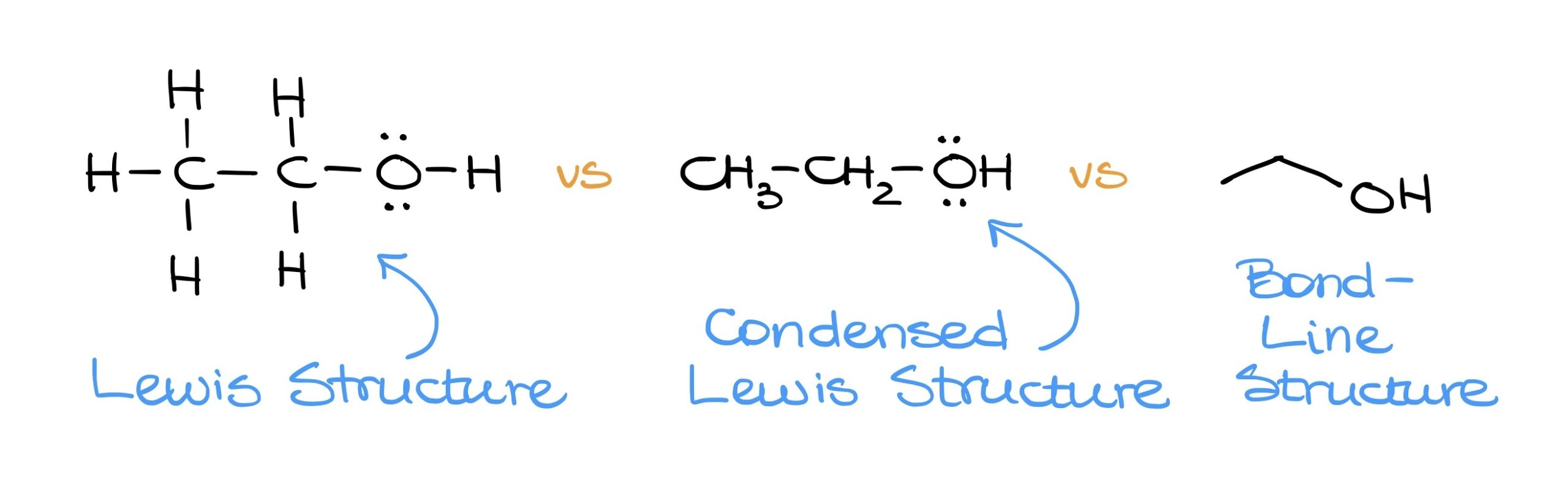
- The full Lewis structure displays every atom and bond.
- The condensed Lewis structure omits some bonds but showcases all atoms.
- The Bond-Line structure is the most streamlined, representing organic molecules through simple lines. This format, while concise, conveys the same information as the other two structures.
Drawing Bond-Line Structures
When transitioning to Bond-Line structures, the focus is primarily on carbon-carbon bonds and bonds between carbons and heteroatoms (like oxygen, nitrogen, sulfur, phosphorus, etc.). The carbon atoms in these structures are implicit, while heteroatoms are always shown.
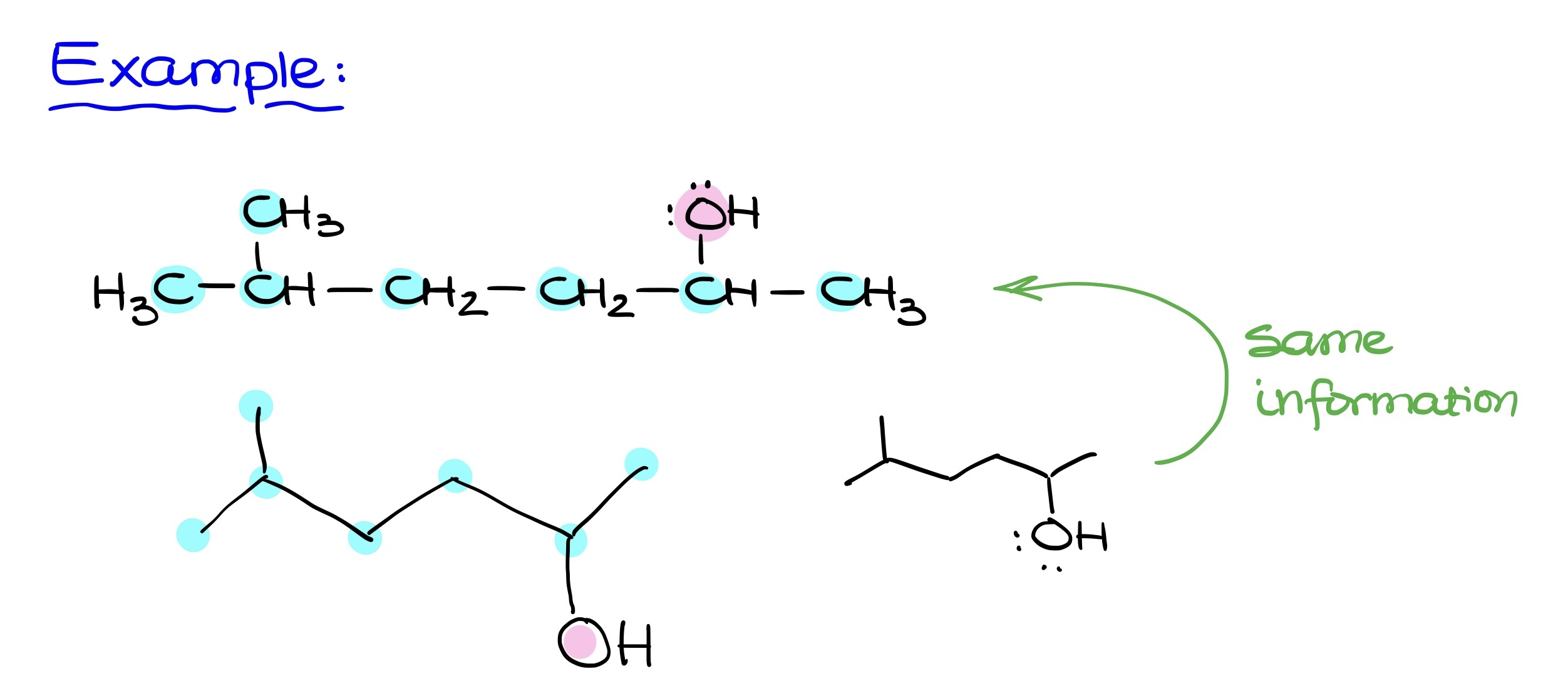
Hydrogens bound to carbon are also implicit, but those attached to heteroatoms must be explicitly shown. Essentially, the core principle involves retaining carbons and the connections between them. It’s crucial to remember that just because some elements like hydrogens and electron pairs are implicit, they are still present in the molecule. Forgetting these implicit hydrogens and electron pairs is a common error.
Do’s and Don’ts of Bond-Line Structures
Drawing Bond-Line structures efficiently requires adhering to certain guidelines. These include:
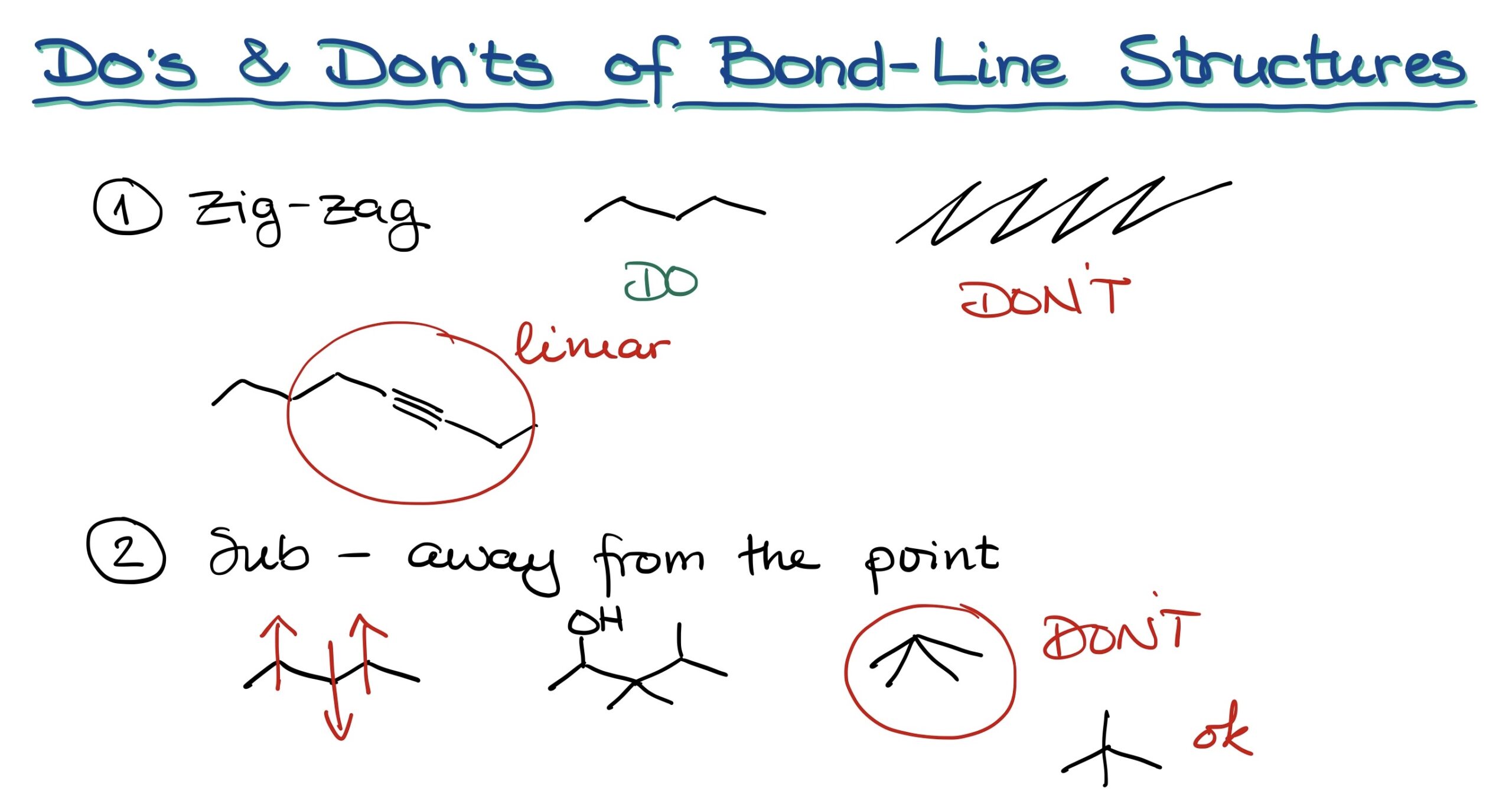
- Zig-zag lines: Always draw structures in neat zig-zags, as shown in [figure 03]. Avoid drawing them like a picket fence or with exaggerated angles.
- Triple bonds should be linear: This guidance stems from the geometry of the sp-hybridized atoms.
- Draw substituents correctly: These should be away from the main point. Do not angle them improperly, which could lead to ambiguities, especially when using dashes and wedges that denote three-dimensional configurations.
Converting Bond-Line Structures back to Lewis Structures
While transitioning from Lewis to Bond-Line structures is common, sometimes the reverse is needed. The process involves:
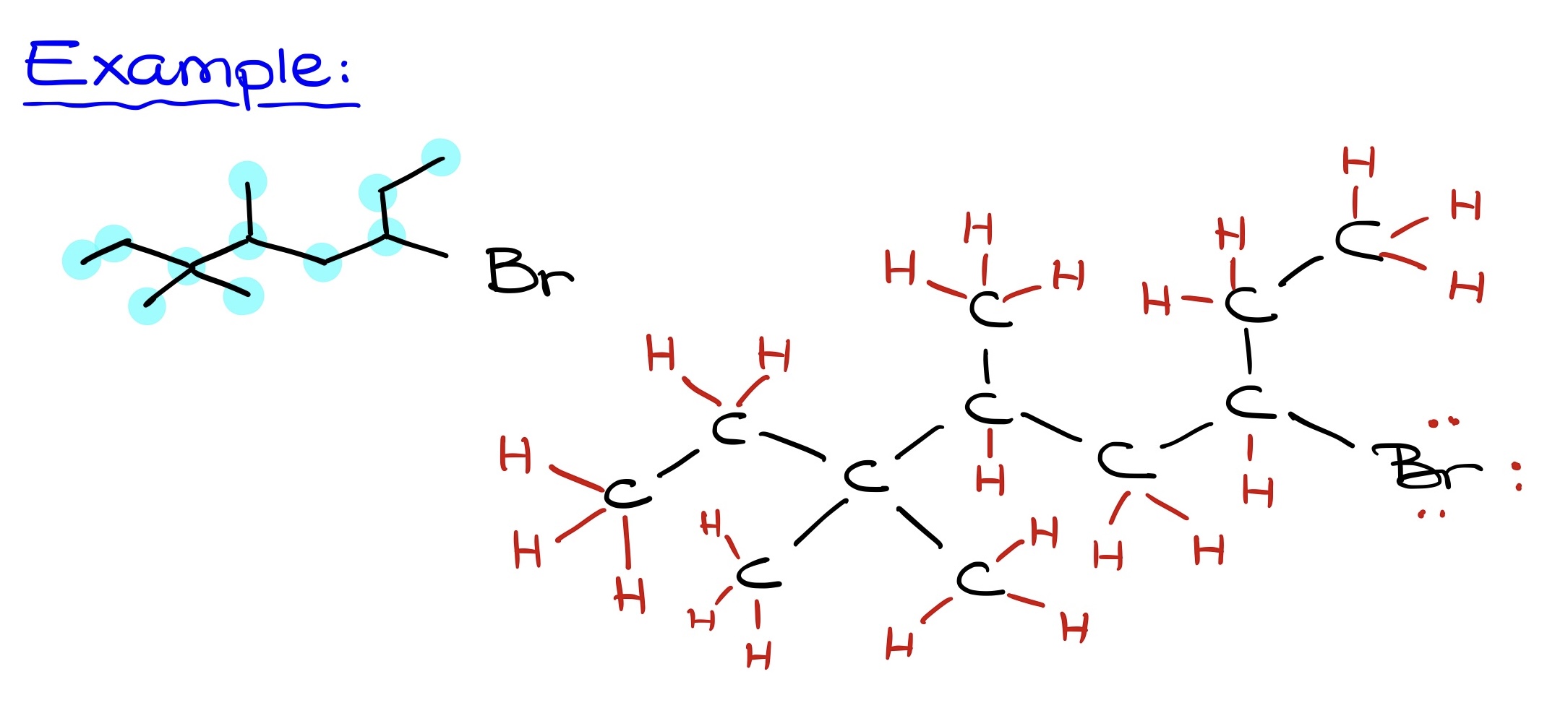
- Identifying the number of carbons: This count determines the molecule’s core structure.
- Identifying other atoms: Bromine, oxygen, nitrogen, etc., should be placed as they appear in the Bond-Line structure.
- Adding hydrogens: Since each carbon requires 4 bonds, the number of hydrogen atoms can be determined by the existing number of bonds in the molecule.
With a firm grasp on these principles, converting between Lewis and Bond-Line structures becomes straightforward.
More Examples
Referencing a Bond-Line structure, begin by identifying all carbons. After charting the skeletal carbon structure, add in heteroatoms like oxygen. Finally, ensure that implicit hydrogens on heteroatoms are explicitly shown. The final step is to draw the molecule neatly, capturing all elements and their respective connections. This streamlined approach offers efficiency without sacrificing accuracy.
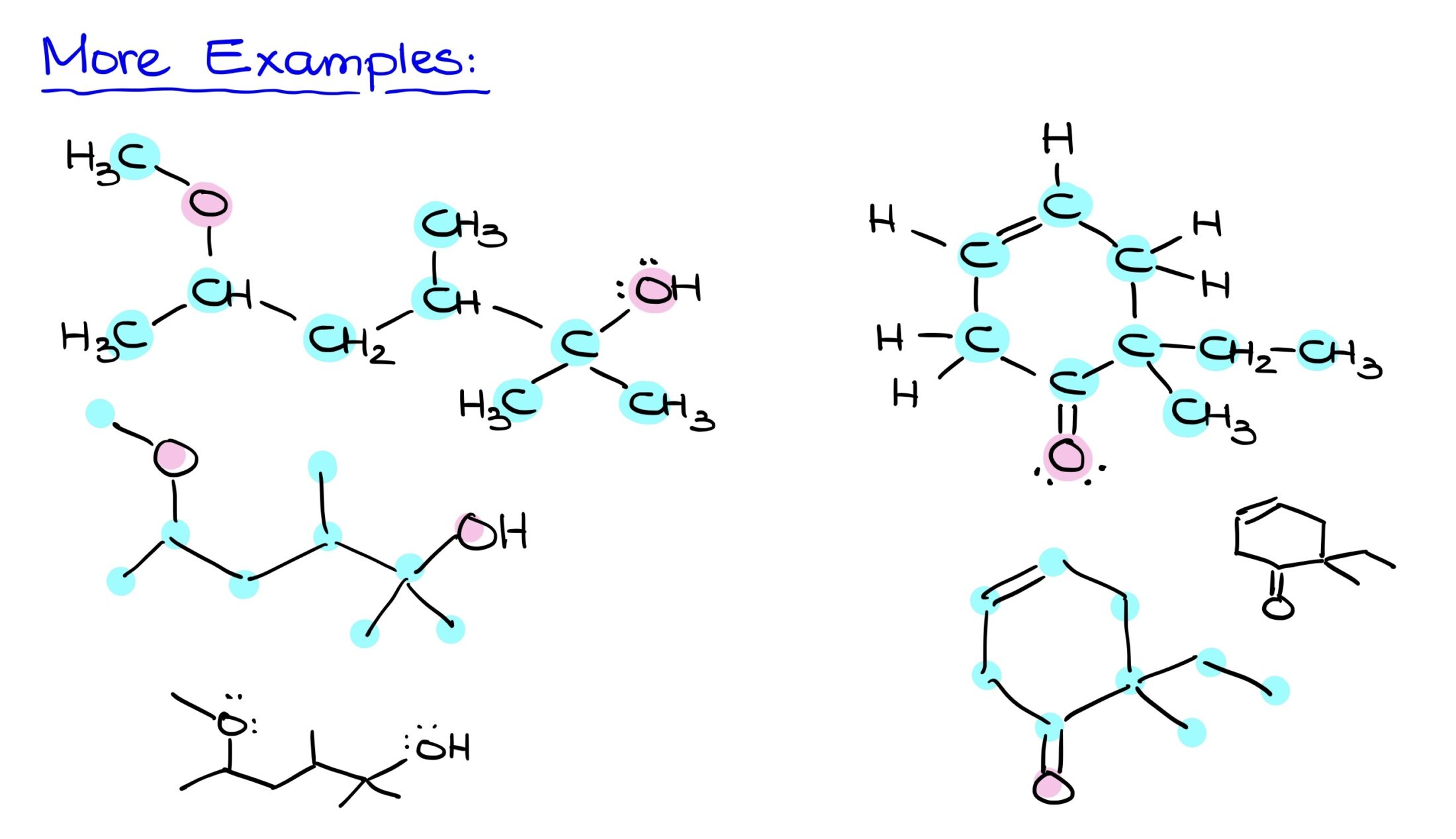
For more practice questions, check out the workbook here. Remember, the key to mastering Bond-Line structures lies in practice and understanding their foundational principles.

hi Victor! at the very end of the video, the embeds to your other videos cover up your illustrations. while i can guess what the final result is, i think it’s good to keep it plainly visible in the video’s end.
You could always pause the video… But yeah, this one is one of my early videos. Later on I started using the outro screen. You can also see the examples from the video in the text below, so you don’t have to worry about catching the exact moment in the video to pause.