Dehydration of Alcohols
The dehydration of alcohols is an elimination reaction which yields an alkene via the water elimination. There are two types of the alcohol dehydration. The E1 method is based on the dehydration of alcohols in acidic media at high temperatures. And the E2 method is based on the conversion of the alcohol functional group into a good leaving group in non-acidic condition followed by the elimination reaction. Each method has its own benefits and drawbacks. In this tutorial we’ll go over the typical conditions for each method, the corresponding mechanisms, and everything you need to know about the dehydration of alcohols for the test.
Dehydration of Alcohols in Acidic Media
We’ll start this review with the dehydration of alcohols in acidic media. This is a fairly typical E1 reaction which proceeds through the formation of the corresponding carbocation intermediate.
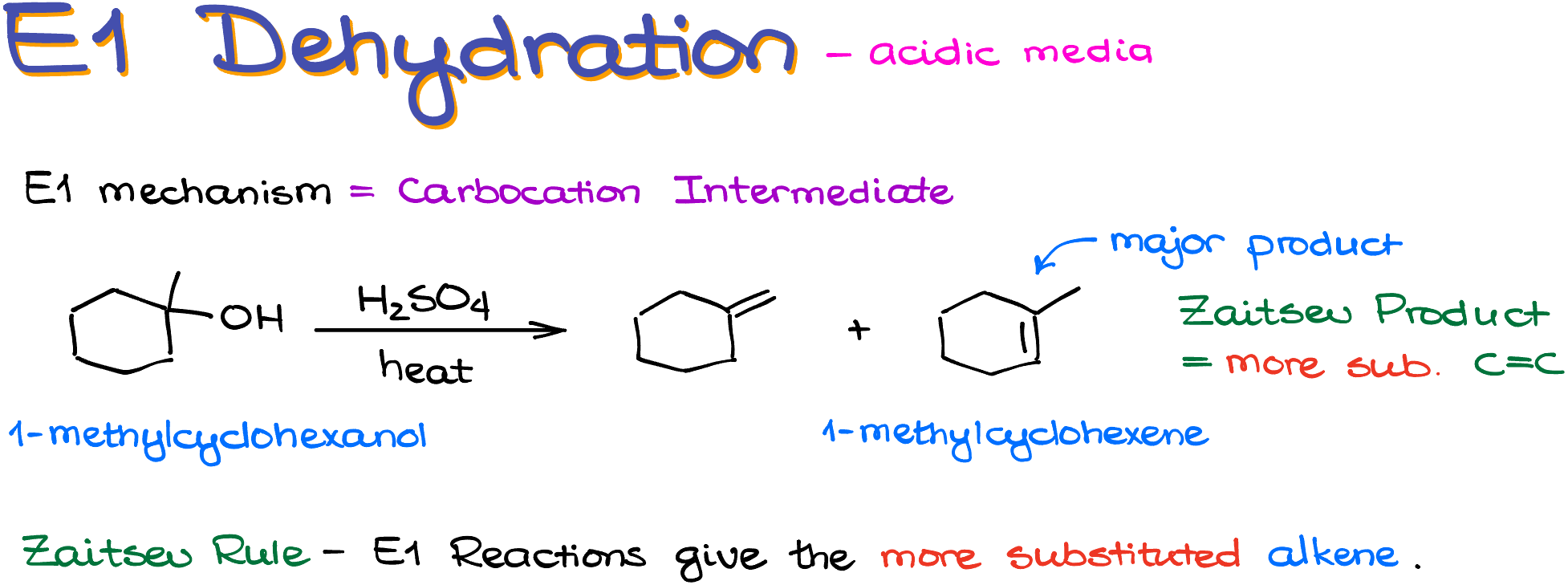
For instance, if we take 1-methylcyclohexanol and heat it with sulfuric acid, we are going to get two isomeric alkene products. The more substituted one, 1-methylcyclohexene, is going to be the major product here. We sometimes refer to the formation of the more substituted alkene in E1 reactions as the formation of the Zaitsev product. And likewise, the tendency of the E1 reactions to form the more substituted alkene is often called the “Zaitsev Rule.”
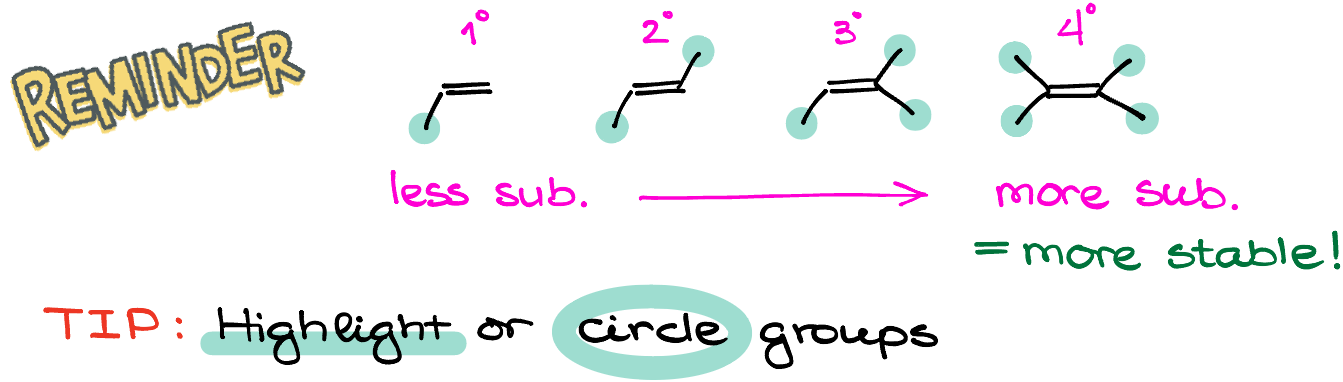
As a quick reminder, let’s go over what exactly it means to be more or less substituted when it comes to alkenes. We look at all non-hydrogen groups connected to our double bond. The more of those groups we have sitting on the alkene, the more substituted it will be. Thus, you can have an alkene with up to four substituents. And for as long as there are no any odd steric effects or any angular torsion that we would see in the case of small rings, more groups on alkene would correlate with the higher stability of the molecule.
The easiest way to see what’s more substituted and what’s less substituted is to simply highlight the carbons (or other non-hydrogen atoms) connected to your double bond and count those. During the test, when the stakes are high, it’s better to spend an extra second highlighting or circling your atoms than to lose points because you missed a connection and mislabeled your alkenes. So, as Richard Feynman famously said: “think with paper!”
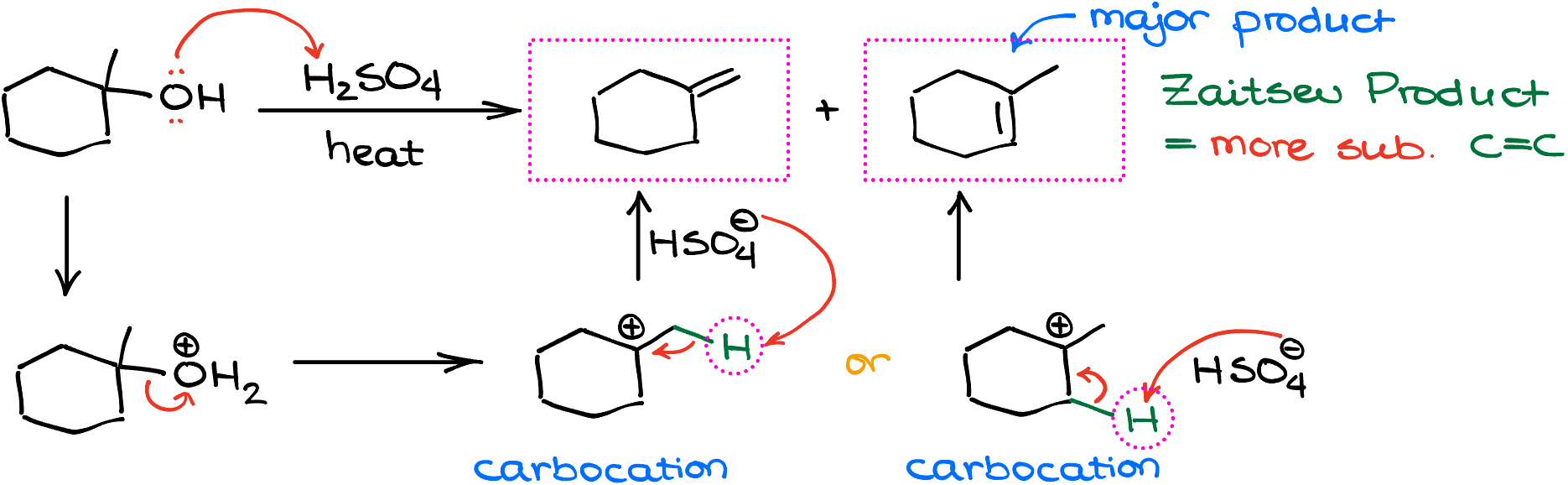
Now, returning back to my mechanism, I’ve mentioned that these reactions form a carbocation intermediates. So, if we go through the mechanism of this reaction, we’ll start by protonating our substrate to make the -OH into a better leaving group. Once we have the H2O sitting on our cycloalkane, it can dissociate leaving a carbocation behind. Since there’s no suitable nucleophile to attack our carbocation, we are going to use the sulfate anion (our conjugate base) or another molecule of the alcohol itself to pull the proton off our carbocation intermediate and form a carbon-carbon double bond. In this example, there are two possible positions from where we can take the hydrogen. Thus, we’re going to end up with two isomeric alkenes as our products.
Carbocation Rearrangements
Since our reaction involves the carbocation intermediates, we need to be careful with the possible carbocation rearrangements. Remember, if a carbocation can rearrange to give you a more stable carbocation, it absolutely will! So, always check your carbocations before proceeding to the next step.
For instance, if I react 3,3-dimethylbutane-2-ol with sulfuric acid at elevated temperature, we’ll end up with two alkene products, none of which will have the same skeleton as the original molecule!
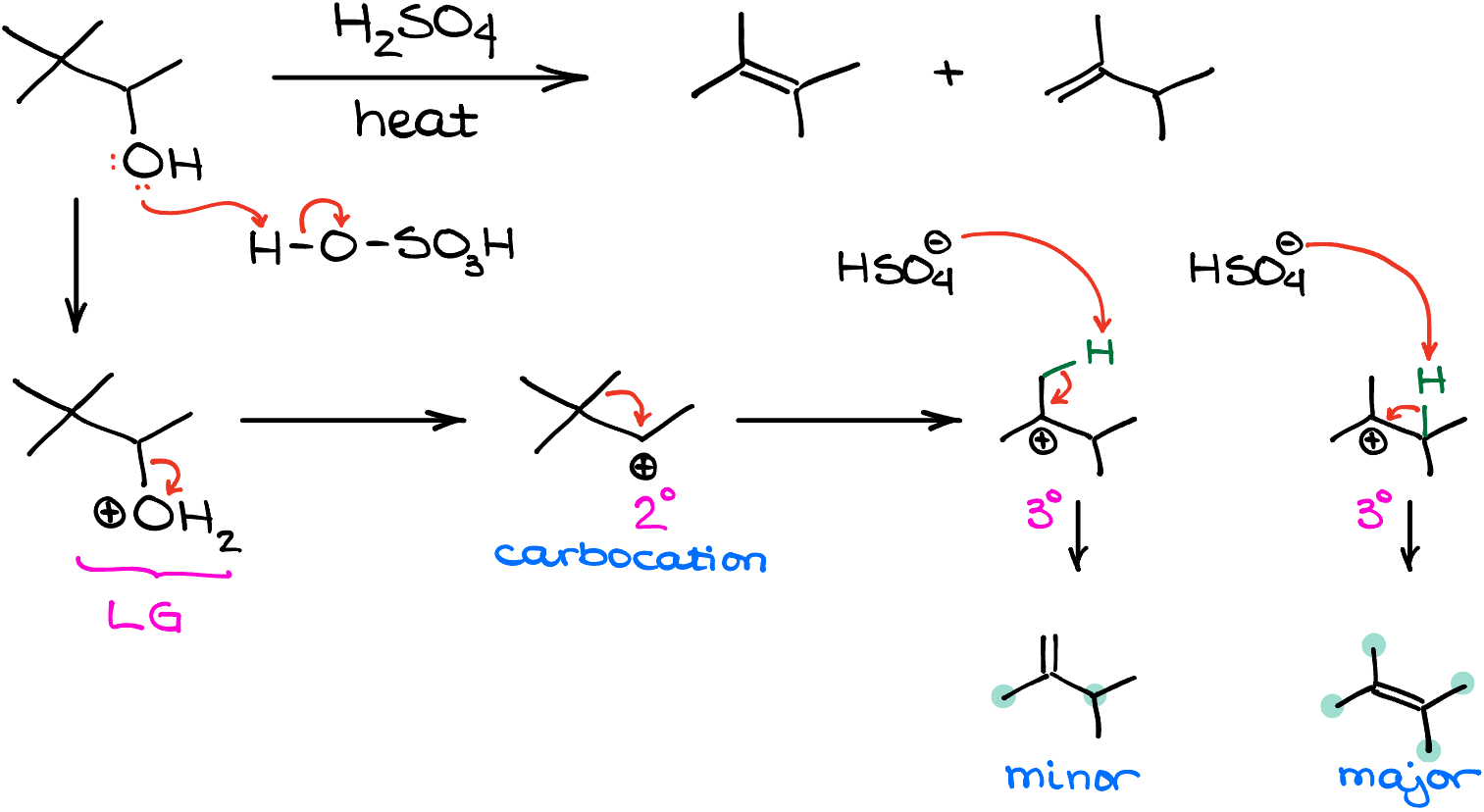
Let’s work through the mechanism of this reaction, so we can see how that happened. Like in the previous example, we first protonate the -OH group. This gives us a good leaving group, that forms a corresponding carbocation intermediate upon dissociation. This secondary (2°) carbocation then undergoes a quick alkyl shift making a more stable tertiary (3°) carbocation. This carbocation has two suitable protons that can be eliminated to make the double bond. Elimination of a proton from the methyl group (-CH3) gives us the minor product, 2,3-dimethylbut-1-ene. And the elimination of a proton from the middle of the molecule, gives us the major product, 2,3-dimethylbut-2-ene. And like in the previous case, I determined the major product by looking at how many carbons I have attached to my double bond. The major product has 4 groups, while the minor product only has 2 substituents on the alkene.
So, as you can see, the E1 dehydration of alcohols is a fairly straightforward reaction what typically gives the most substituted alkene as the major product. The only caveat here is the possibility of the carbocation rearrangements. So, double check your intermediates when you’re working through the mechanism. And I do suggest that you always jot down the mechanism in broad strokes for all of your reactions. If you don’t “visualize” your carbocation and its structure, you may easily miss the carbocation rearrangement and end up predicting the incorrect product.
E2 Alcohol Dehydration
If your molecule is sensitive to acids, or if you want to avoid the carbocation rearrangements, or you need more control over your reactions, then you should look into the E2-style dehydrations. The general idea of those is going to be to convert your alcohol into a good leaving group and eliminate it with some sort of a base base. We can do it in two separate steps or we can do it in a single synthetic procedure. Of course, the less steps you have, the more convenient it’s going to be. So, here I’m going to show you one of the most common gentle E2 reactions of alcohols using the phosphorous oxychloride as the activating agent in the presence of pyridine.
The reaction starts with the alcohol attacking the phosphorus and displacing one of the chlorines. This is a typical SN2 reaction. Then, we do a proton transfer to make a neutral intermediate. At this point, instead of the -OH, we have an excellent leaving group. And since we’re working in pyridine as a solvent, we’ll have the next equivalent of pyridine come in and do a typical E2 reaction giving us the alkene product. As you can see, in this case, we have no carbocation rearrangements or any similar complications!
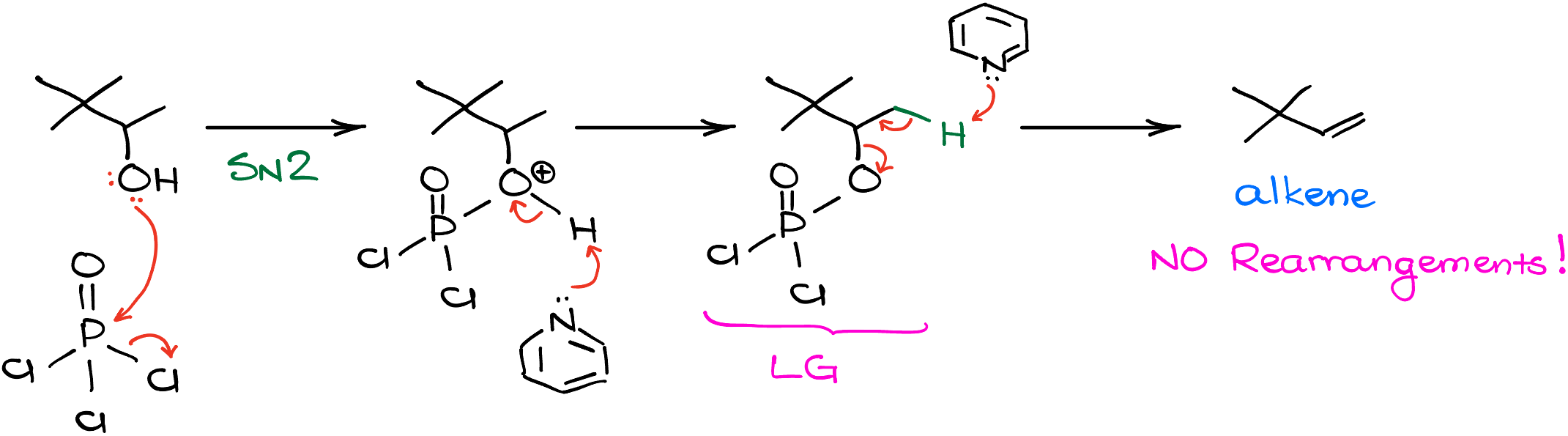
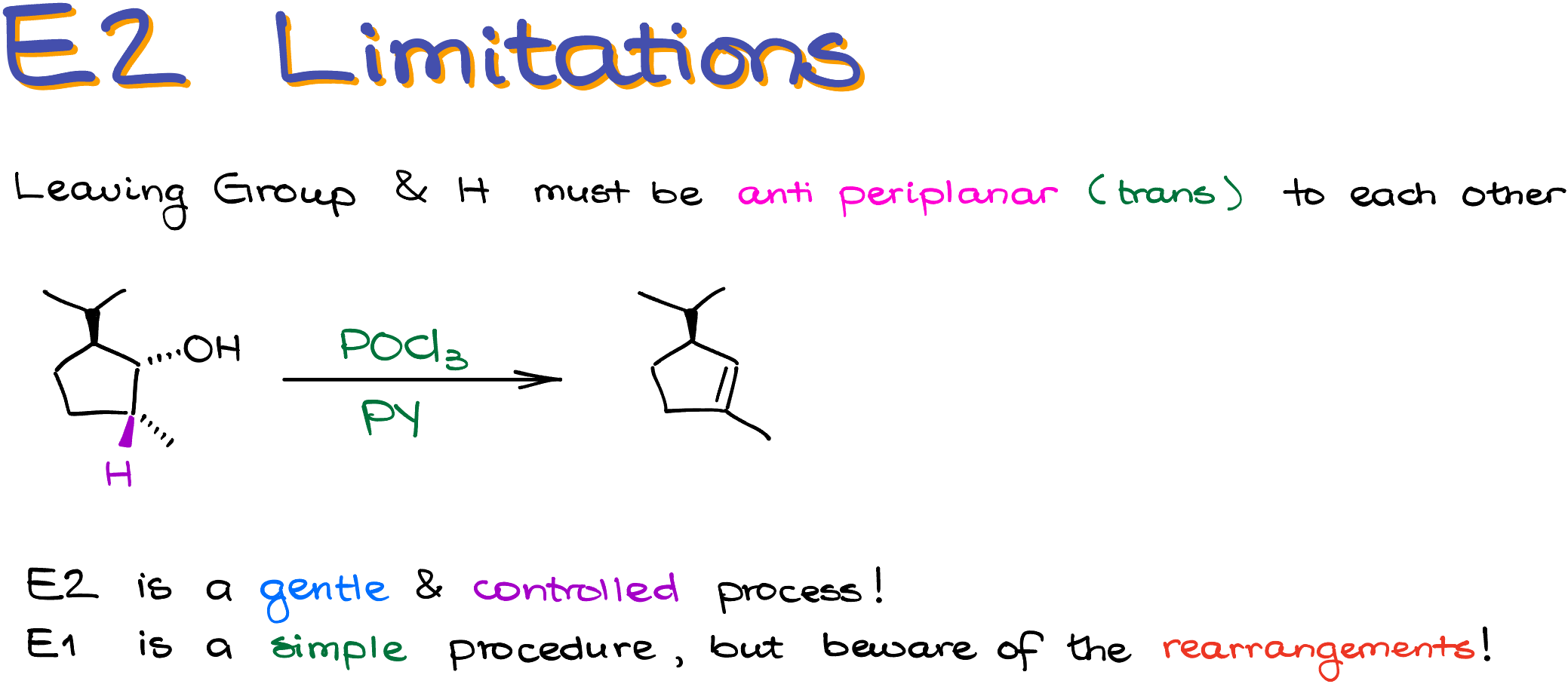
E2 Dehydration Limitations
And since this is an E2 reaction, we’re going to have all the same limitations that a typical E2 would have. Namely, the leaving group and the hydrogen must be anti-periplanar to each other for the reaction to happen. This reaction also tends to give the Zaitsev product when possible. Although, there are modification that give the Hofmann product, that goes beyond the scope of this tutorial, so I’m not going to review those here.
Let me illustrate the anti-periplanar limitation with an example. For instance, in this reaction, the only hydrogen that would be available for the elimination is the one on the same carbon with the methyl group. So, our product will have the alkene going down from the carbon where the -OH used to be.
Since E2-style dehydrations proceed in much gentler conditions than the E1 reactions, they tend to be more tolerant to other functional groups you might have in your molecule. Which is an important consideration when you’re planning your synthesis.
Concluding Thoughts
Overall, the dehydration of alcohols via either E1 or E2 pathways, is a typical elimination reaction. So, for as long as you understand how those reactions work, you should be able to predict the outcomes of the alcohol dehydration reactions fairly easily.
