Decarboxylation of Carboxylic Acids
In this tutorial we’re going to talk about the decarboxylation of carboxylic acid. Whenever we talk about decarboxylation, we’re focusing on the removal of a carboxylic acid group, typically in the form of carbon dioxide gas, or CO₂.
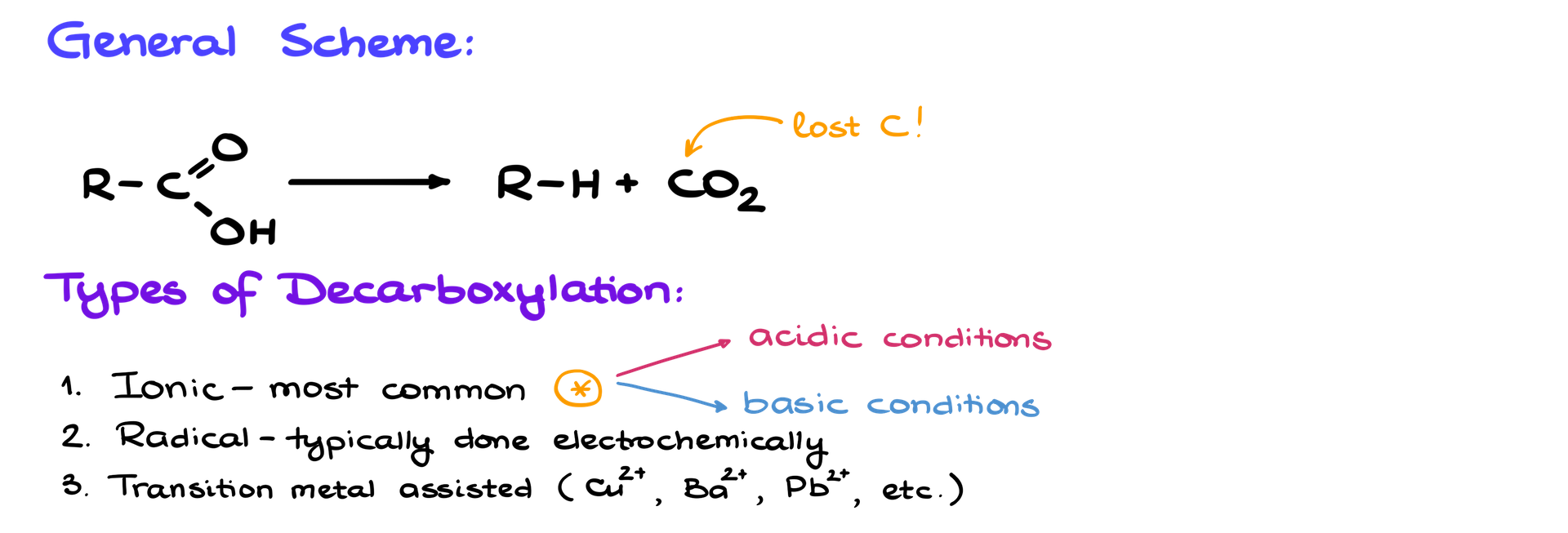
There are a few types of decarboxylation reactions you might encounter. The most common, and the one we’ll focus on today, is ionic decarboxylation. However, you might also come across radical decarboxylation, which usually happens electrochemically or at very high temperatures, or metal-assisted decarboxylations, where heavy metals like copper or barium help out. These last two types are less useful for practical synthesis and tend to require extreme conditions. That’s why today, we’ll stick to the ionic kind, which is much more manageable.
Decarboxylation in Basic Conditions
Let’s start with decarboxylation under basic conditions. If you take a carboxylic acid and treat it with a base, the first thing that happens is a simple acid-base reaction. The base snatches the proton off the carboxylic acid, leaving you with a carboxylate ion. At this point, the carboxylate can lose CO₂, leaving behind the R-group with a negative charge and some CO₂ gas that floats away.
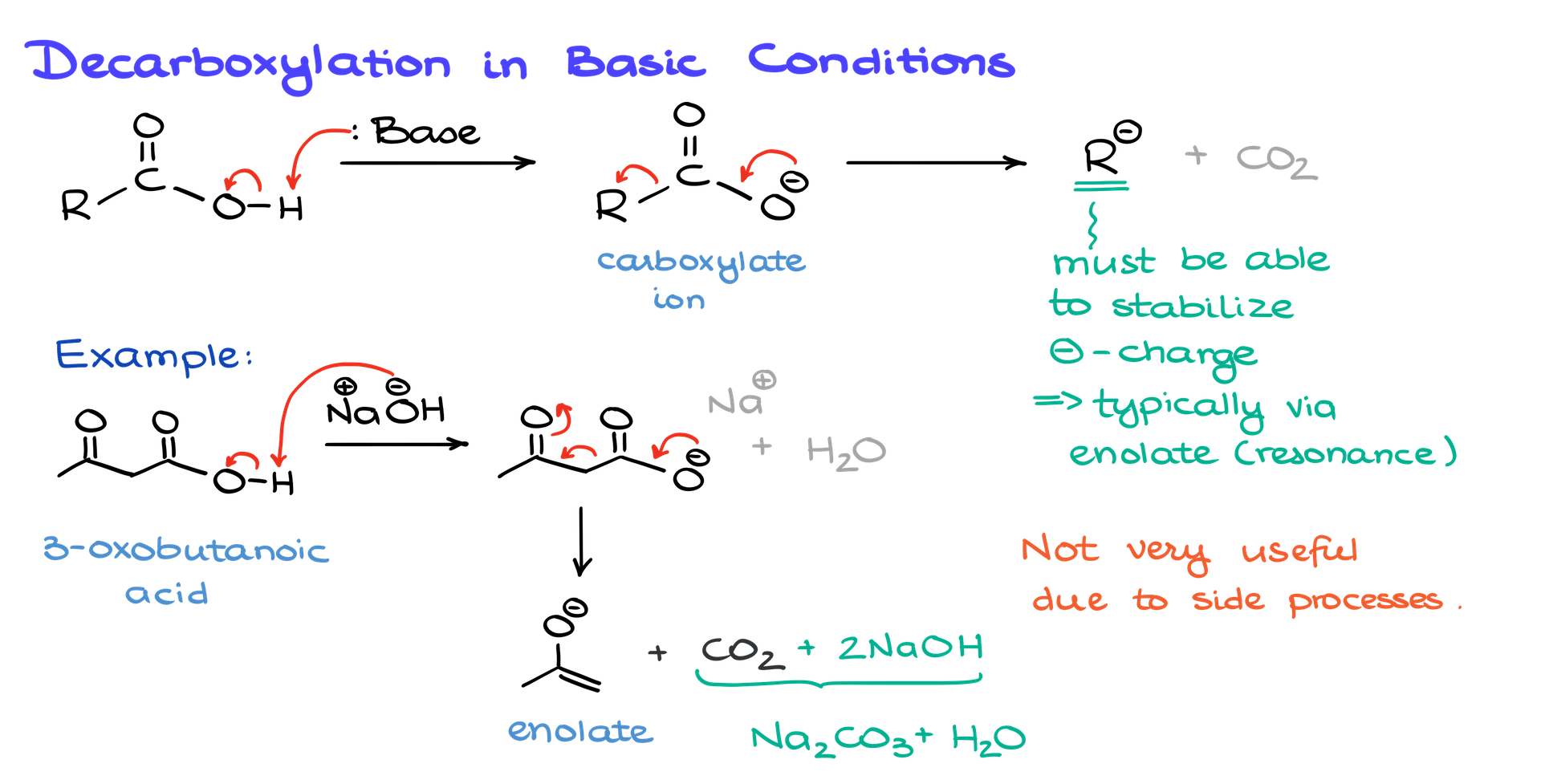
Here’s the catch, though: not every R-group is cut out for this. For the reaction to work, that R-group needs to stabilize the negative charge left behind, often through resonance. A plain alkyl chain won’t do. Instead, you’re looking at something like an enolate ion, which has the structure to handle the charge.
For example, if you take acetoacetic acid (or 3-oxobutanoic acid if you prefer its IUPAC name) and treat it with a base like sodium hydroxide, the base deprotonates the acid to form the carboxylate ion. That ion can then lose CO₂, leaving behind an enolate ion. Sounds good, right? Unfortunately, this reaction comes with problems.
First, the CO₂ reacts with the sodium hydroxide, forming sodium carbonate and using up your base. This means you’d need an excess of sodium hydroxide to keep the reaction going. Second, the carbonyl group in the molecule can react with the base, derailing the whole process. And if that wasn’t enough, the enolate ion is highly nucleophilic, so it might jump into other side reactions you don’t want. In short, basic decarboxylation is messy, impractical, and usually not worth the trouble.
Decarboxylation in Acidic Conditions
Now let’s shift gears to decarboxylation in acidic conditions, which is much more reliable and useful. This works especially well for β-keto carboxylic acids—carboxylic acids where the carbonyl group is in the β position relative to the carboxylic acid. These reactions can occur with just mild heating, usually between 50 and 150°C, and sometimes even spontaneously at room temperature.
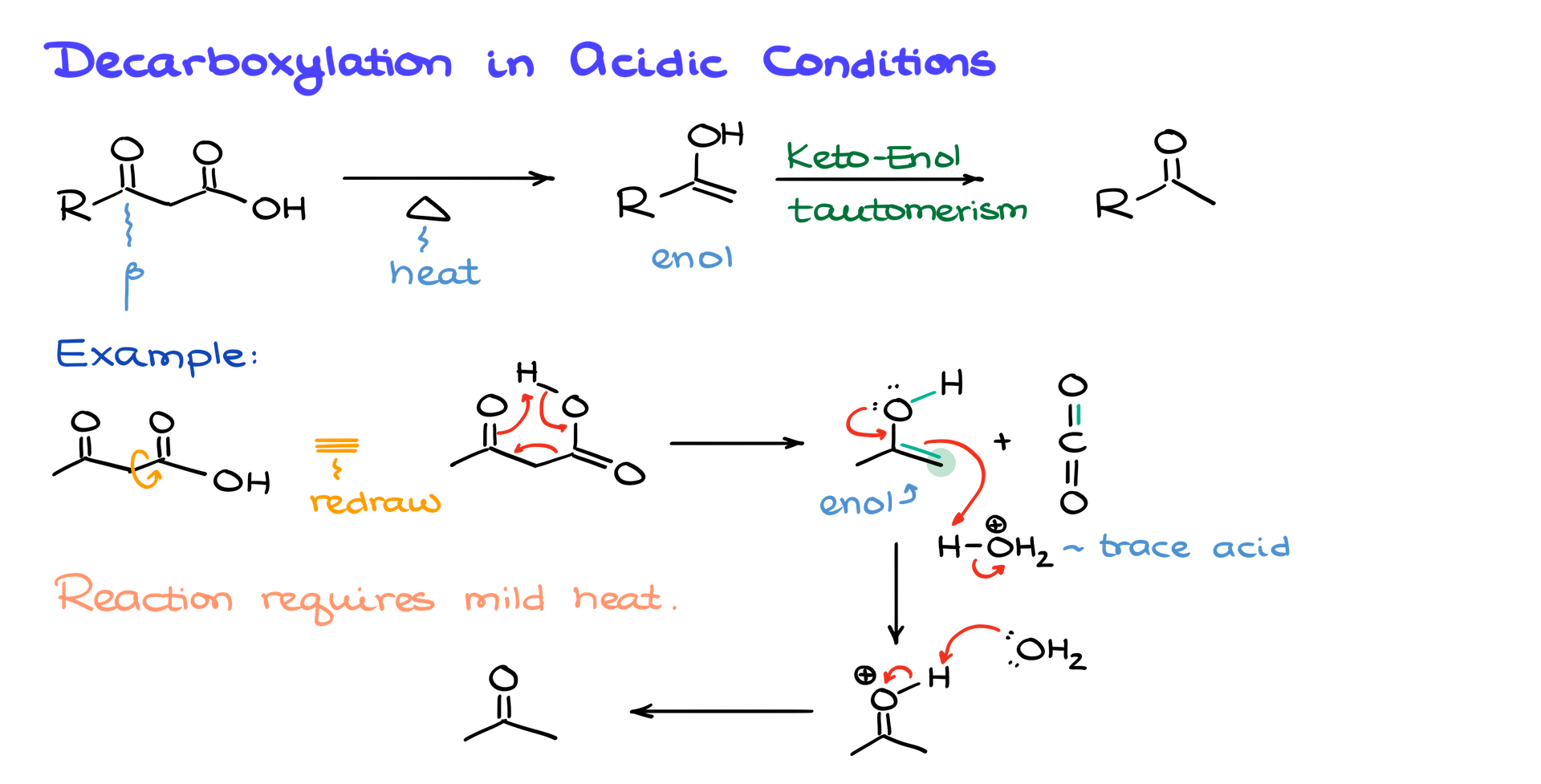
Here’s how it works. When you heat a β-keto carboxylic acid, it first forms an intermediate called an enol. This enol then undergoes keto-enol tautomerization, which rearranges the molecule into a more stable carbonyl compound. For example, if you start with acetoacetic acid, mild heat is enough to kick off decarboxylation, producing CO₂ gas and an enol intermediate. That enol quickly tautomerizes into acetone, your final product. The beauty of this reaction is its simplicity and the fact that it only requires mild conditions.
Stability of Carboxylic Acids
One thing to keep in mind is the stability of your starting materials. Regular carboxylic acids, where the functional group is attached to a simple alkyl chain, are stable and won’t decarboxylate spontaneously. However, as soon as you introduce something that can stabilize the negative charge—like a carbonyl group in the β position—things change. These β-keto carboxylic acids are unstable and will decarboxylate at elevated temperatures. If you add a really strong stabilizer, like a halogen, the molecule might not even last at room temperature.
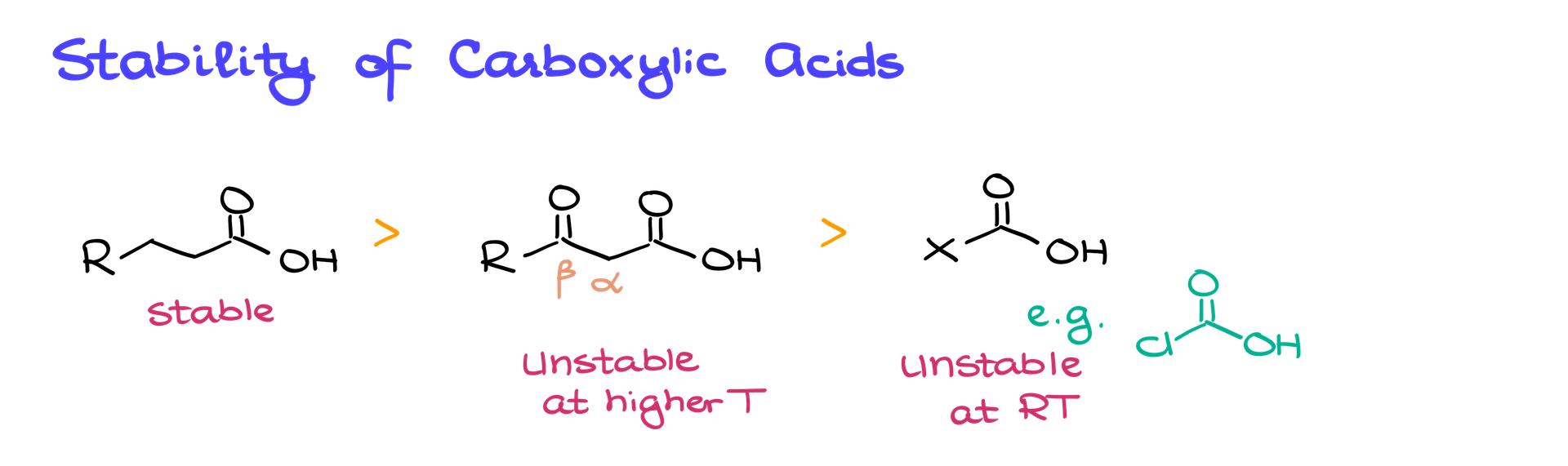
This is critical for synthesis. Unstable carboxylic acids, like β-keto carboxylic acids, usually show up as intermediates rather than starting materials. If you’re planning a synthesis and include something unstable in your starting lineup, it’s not going to sit there waiting for you—it’ll decompose on its own.
Shortcut for Predicting Products
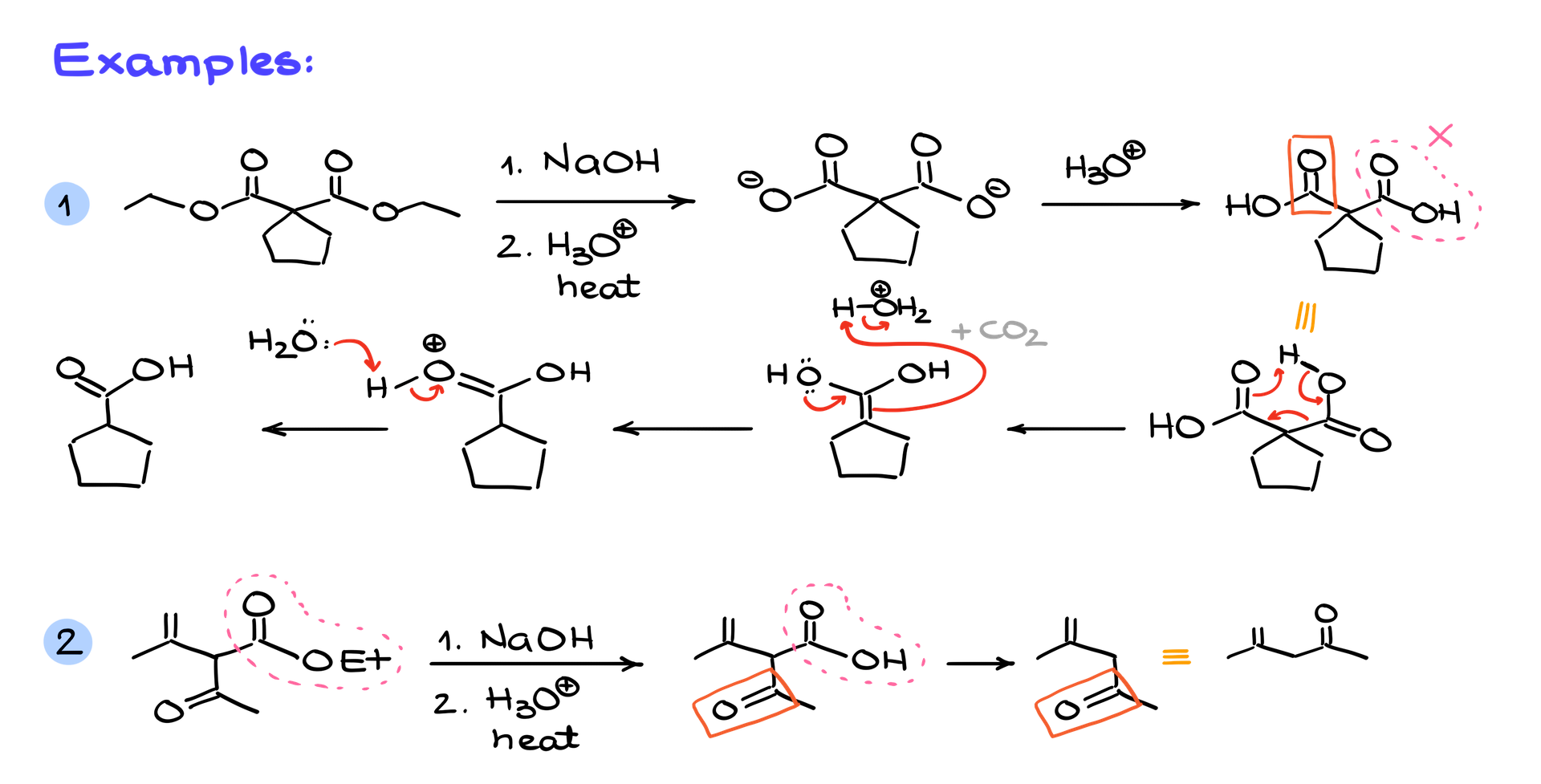
Here’s a quick trick to predict the product of a decarboxylation reaction. If you have a β-keto carboxylic acid, just erase the carboxylic acid group and keep the rest of the molecule intact. For instance, if you’re starting with a β-keto ester, convert it to the corresponding β-keto carboxylic acid and then remove the carboxylic acid group to get your product. No need to work through the whole mechanism unless you’re asked to—just focus on what’s left after the CO₂ leaves.
Final Thoughts
Decarboxylation is an important reaction, but it’s also sneaky. β-Keto carboxylic acids are unstable and can undergo spontaneous decarboxylation, especially when heated. This means you need to be careful when designing synthetic pathways or interpreting reaction conditions. If you see an instructor hinting at elevated temperatures on an exam, they’re probably testing your ability to spot this reaction. Stay sharp, watch for unstable intermediates, and you’ll do just fine.
