Cope Elimination
In this tutorial, I want to talk about the Cope elimination, which uses a nitrogen-based leaving group to force a syn elimination, giving us the corresponding alkene. So let’s look at the mechanism of this reaction.

Mechanism of the Cope Elimination
The mechanism of the Cope elimination consists of two distinct phases.

In the first part, we carry out the oxidation reaction. The starting point for the Cope elimination is always a tertiary amine, where the nitrogen atom is typically connected to two methyl groups, although other examples have been reported. As our oxidation agent, we use MCPBA, which you might remember from the epoxidation of alkenes or perhaps Baeyer-Villiger oxidation.
The first step involves the nitrogen, being a good nucleophile, attacking the oxygen, breaking the oxygen-oxygen bond, and giving us the following intermediate. Then, the carboxylate floating around deprotonates our intermediate, leading to the formation of our product, which we refer to as either an amine oxide or simply an oxide.
There is, however, an alternative oxidation technique you might see in your course, which involves hydrogen peroxide. Mechanistically, this oxidation is quite similar. The nitrogen performs a nucleophilic attack on the oxygen of the hydrogen peroxide, breaking the oxygen-oxygen bond and forming an intermediate. As in the previous case, we then deprotonate—this time with hydroxide—yielding our final product: a nitrogen oxide or amine oxide.
Elimination Portion of the Cope Elimination (syn Elimination)
Once we have our oxidation product, we move on to the next part of the mechanism: the elimination itself. An interesting aspect of this elimination is that the nitrogen-containing group acts as both the leaving group and the base at the same time. The process works as follows: I’ll indicate the protons involved, and the negatively charged oxygen will abstract one of those protons, triggering a cascade of electron movement. This results in the breaking of the carbon-nitrogen bond and the formation of our final product: an alkene. The byproduct is hydroxylamine, which we typically disregard.
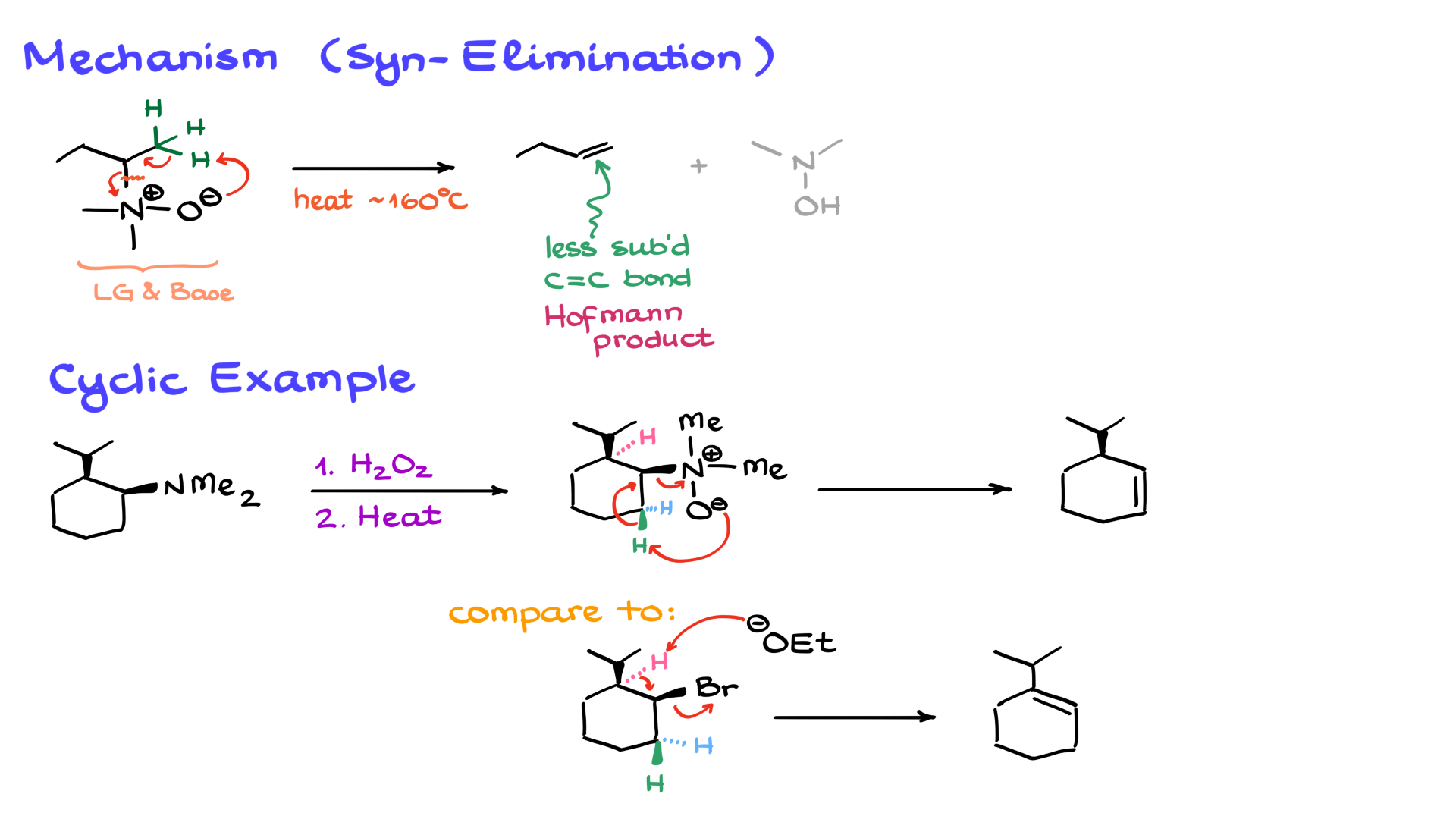
A few important things to keep in mind about this reaction: This step is not spontaneous. It requires a significant amount of heat—typically between 160 to 180°C—which could be detrimental to some molecules, as not every organic substance can withstand such temperatures. Another key point is that the reaction tends to produce the less substituted alkene, known as the Hofmann product. Finally, as I mentioned, this is a syn elimination, meaning the leaving group on the nitrogen oxide and the hydrogen must be on the same side of the molecule. This becomes particularly clear when dealing with cyclic molecules.
Let’s look at an example with a cyclic molecule. Here, I have my amine and some other substituents on the ring. The first step is the oxidation of the nitrogen, so I’ll go ahead and draw the oxidation product. Now, at this point, we have several different hydrogens in the β-position. I’ll draw all of them and color-code them for clarity. In this case, the green hydrogen is on the wedge, the same as the base/leaving group, making it the syn hydrogen. The blue and pink hydrogens, on the other hand, are anti-hydrogens. Since this is a syn elimination, the oxygen specifically abstracts the green hydrogen, as it is the only syn hydrogen available, leading to the final product.
Now, let’s compare this to a typical elimination reaction where our leaving group is a simple halide, such as bromine. The same set of hydrogens is present, but in an E2 reaction, the leaving group must be anti to the hydrogen. That means we would target the blue or pink hydrogen instead. If I introduce a base, it would abstract one of those anti-hydrogens, leading to a different product. While I could attempt to favor the formation of the less substituted alkene by using a bulky base, I would still get a mixture of products. This makes the Cope elimination much more efficient, as it only has one possible pathway: syn elimination. Since there is only one accessible hydrogen for elimination, the reaction gives a single product, allowing for precise control over the outcome.
Because of its strict syn elimination requirement, the Cope elimination offers a powerful tool for fine-tuning reactions in synthesis. Have you ever come across the Cope elimination before? Let me know in the comments below!
Practice Questions
Would you like to see the answers and check your work? Become a member today or login if you’re already a member and unlock all members-only content!

