Claisen Rearrangement
In this tutorial, I want to talk about the Claisen rearrangement, which is a thermal equilibrium process that occurs in allyl vinyl ethers. Essentially, any molecule with this general structure like what I have below can undergo the Claisen rearrangement, as long as it can curl into a cyclic intermediate.
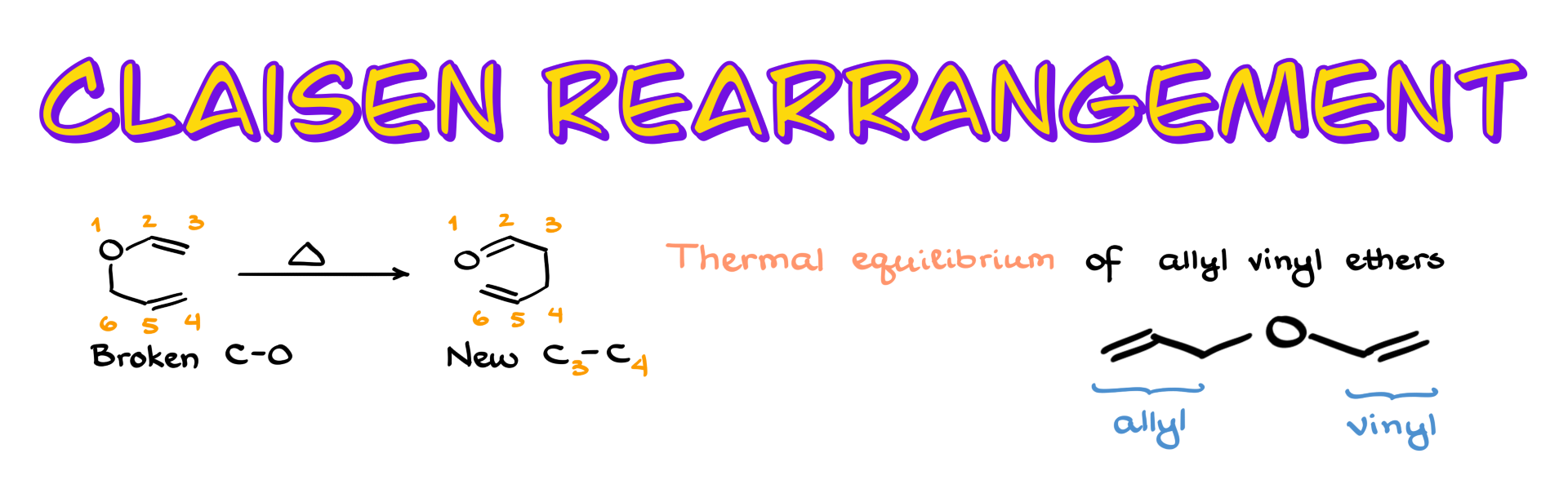
So looking at my starting material, I’m going to number the atoms both in the starting structure and in the product. If I pay close attention to what happens here, I can see that the bond between the carbon and the oxygen is broken, and a new bond is formed between carbons 3 and 4. Also, the π bonds shift positions. This reaction looks very similar to another sigmatropic rearrangement you might have already seen—the Cope rearrangement—because, in both cases, we break one sigma bond, form another one, and the π bonds shift around the molecule.
Mechanism of the Claisen Rearrangement
Mechanistically, the reaction is very similar to the Cope rearrangement.
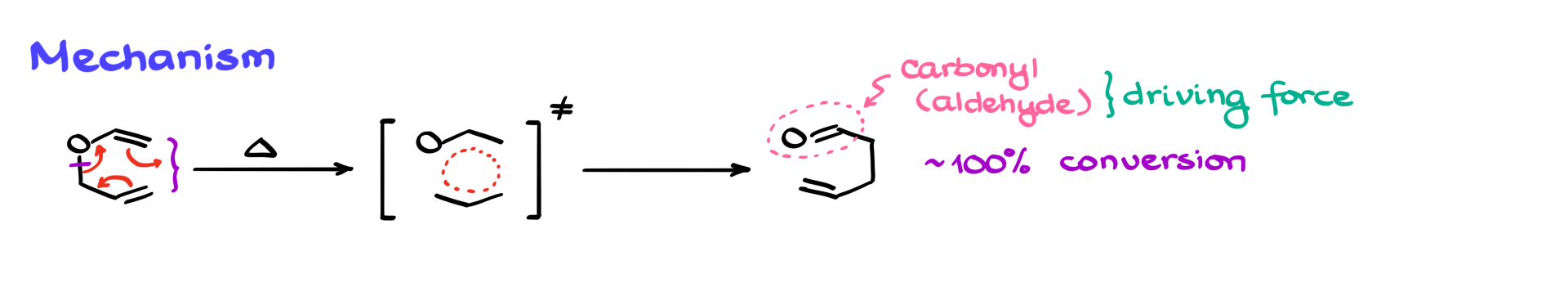
We begin with our starting material, and since this is a pericyclic reaction, we’ll have a cyclic transition state. Because it’s a pericyclic reaction with a cyclic transition state, the way we draw our curved arrows doesn’t really matter. So I’ll show my arrows this way, like that. As a result, we’ll form a new bond between the edge atoms, and we’ll break the bond between the carbon and oxygen, eventually giving us this product. And of course, the π bonds are rearranging as well.
Now, while this reaction is technically a thermal equilibrium like the Cope rearrangement, there’s one major difference. In the Claisen rearrangement, we always end up forming a carbonyl group—specifically an aldehyde in this example. That formation of the aldehyde drives the reaction forward because, thermodynamically, the C=O double bond is significantly more stable than a C=C double bond. This means that, unlike the Cope rearrangement, the Claisen rearrangement typically goes to completion, with nearly 100% conversion and almost no starting material left.
Examples of the Claisen Rearrangement
Now, theory is great, but let’s look at some examples.
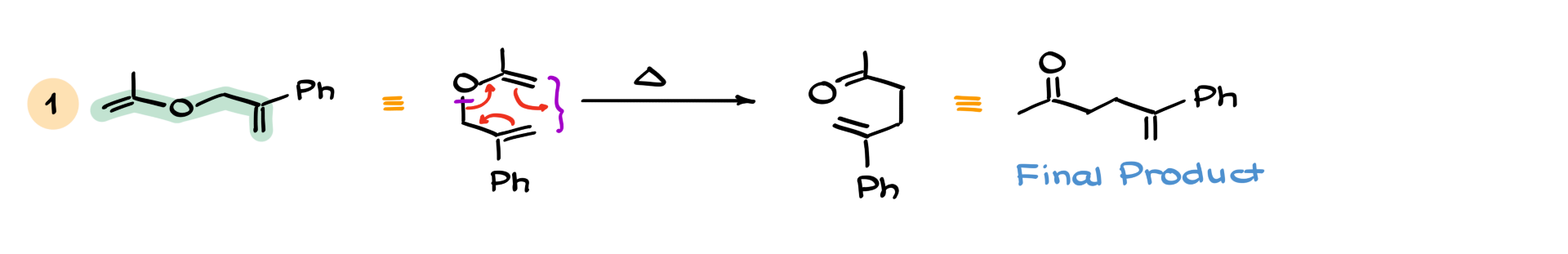
In the first example, we have this molecule here. And as I’ve said many times before, molecules are not always drawn in the most convenient or recognizable form. So it’s up to us to figure out if the molecule can react. We might have to rotate or adjust it into a different conformation to get the reactive shape.
In this particular case, the reactive portion is right here—the left part is the vinyl group, and the right part is the allyl group. If I change the conformation and redraw it the way we’re used to seeing it, we’ll get something like this. Now, when I draw my curved arrows—this goes here, this goes there, and that moves here—we end up forming a new carbon-carbon bond between those two atoms and breaking the bond between carbon and oxygen. That gives us our final product.
Of course, if I wanted to draw this product in a more aesthetically pleasing, linear fashion, it would look like this. So, neither the starting material nor the product has to be in that semicircular, curled shape. Your instructor might just give you both as linear structures and ask you for the mechanism. If you don’t recognize that the molecule can curl into that cyclic transition state, you might not know what to do.
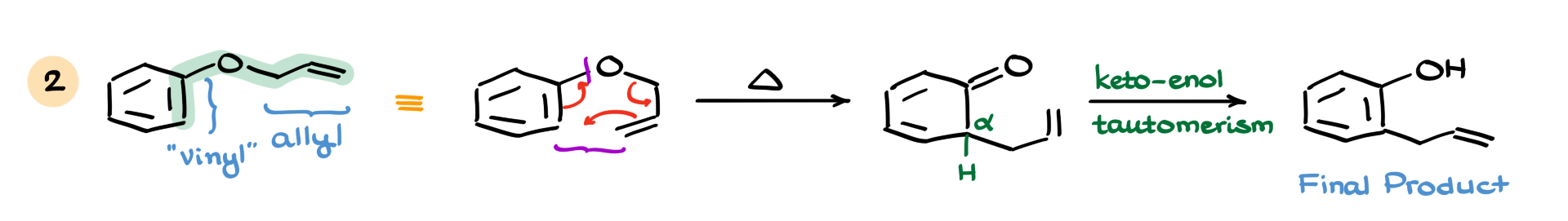
Now let’s check out another example. In this case, the reactive portion is over here. The right side is the allyl part, and the left side is the vinyl part—though I’m putting “vinyl” in quotation marks because it’s actually part of an aromatic ring. And although aromatic rings are incredibly stable, they can still participate in this reaction without too much trouble.
Just like before, I’ll redraw the molecule in a slightly easier form to show the transformation. Then, drawing the curved arrows—this goes here, that goes there—we form a new carbon-carbon bond between these two atoms and break a carbon-oxygen bond here, giving us the following intermediate.
Now, the big thing here is that we’ve broken the aromaticity, which is a big deal. But even though aromaticity is lost temporarily, we still have a hydrogen in the α-position, right next to the carbonyl. That allows us to undergo keto-enol tautomerism, which restores the aromaticity. This gives us a phenol with an allyl group in the ortho position as the final product. So while aromatic rings are usually off-limits in many reactions, in this case, they’re fair game.

Let’s take a look at one more example. Here, the reactive portion is this highlighted section. The allyl group is on the right, and the vinyl group is on the left. Again, to make things simpler, I’ll redraw it in a more cyclic form. From here, I can show the electron movement—this goes here, this moves there, and that shifts here. We’ll form a new carbon-carbon bond, break a carbon-oxygen bond, and end up with this product.
And like the first example, if I wanted to redraw this in a more linear form, it would look like this. Interestingly, we didn’t get an aldehyde or a ketone this time—we ended up with an amide as the final product.
