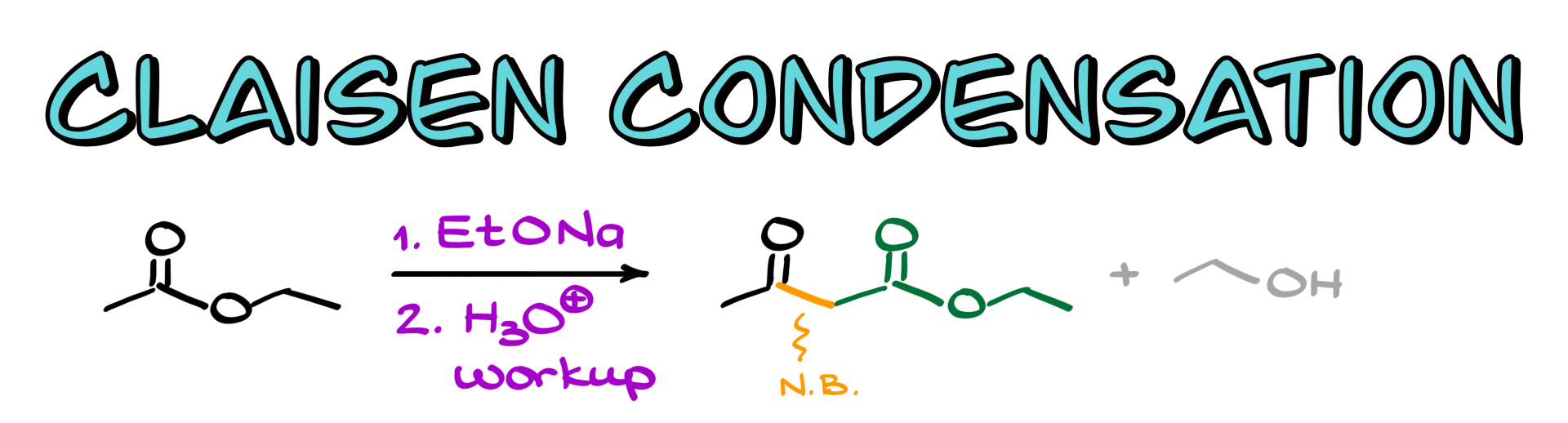
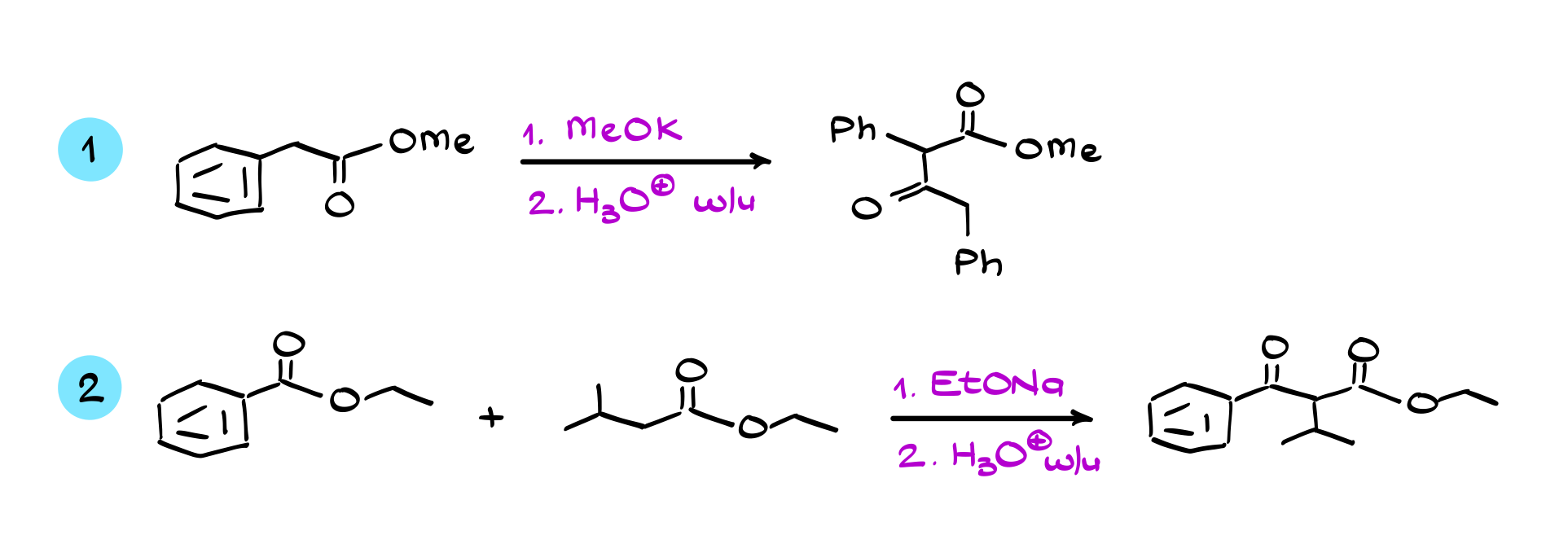Claisen Condensation
In this tutorial, I want to talk about the Claisen condensation, which is a reaction where two equivalents of the same ester, or sometimes two different esters or an ester and another carbonyl compound, combine to produce a 1,3-dicarbonyl compound as the final product.
Claisen Condensation Mechanism
From a mechanistic perspective, the reaction is relatively straightforward. We start with an ester, using ethyl acetate as an example, and the very first step is to deprotonate the alpha position to form the corresponding enolate, which looks like this.
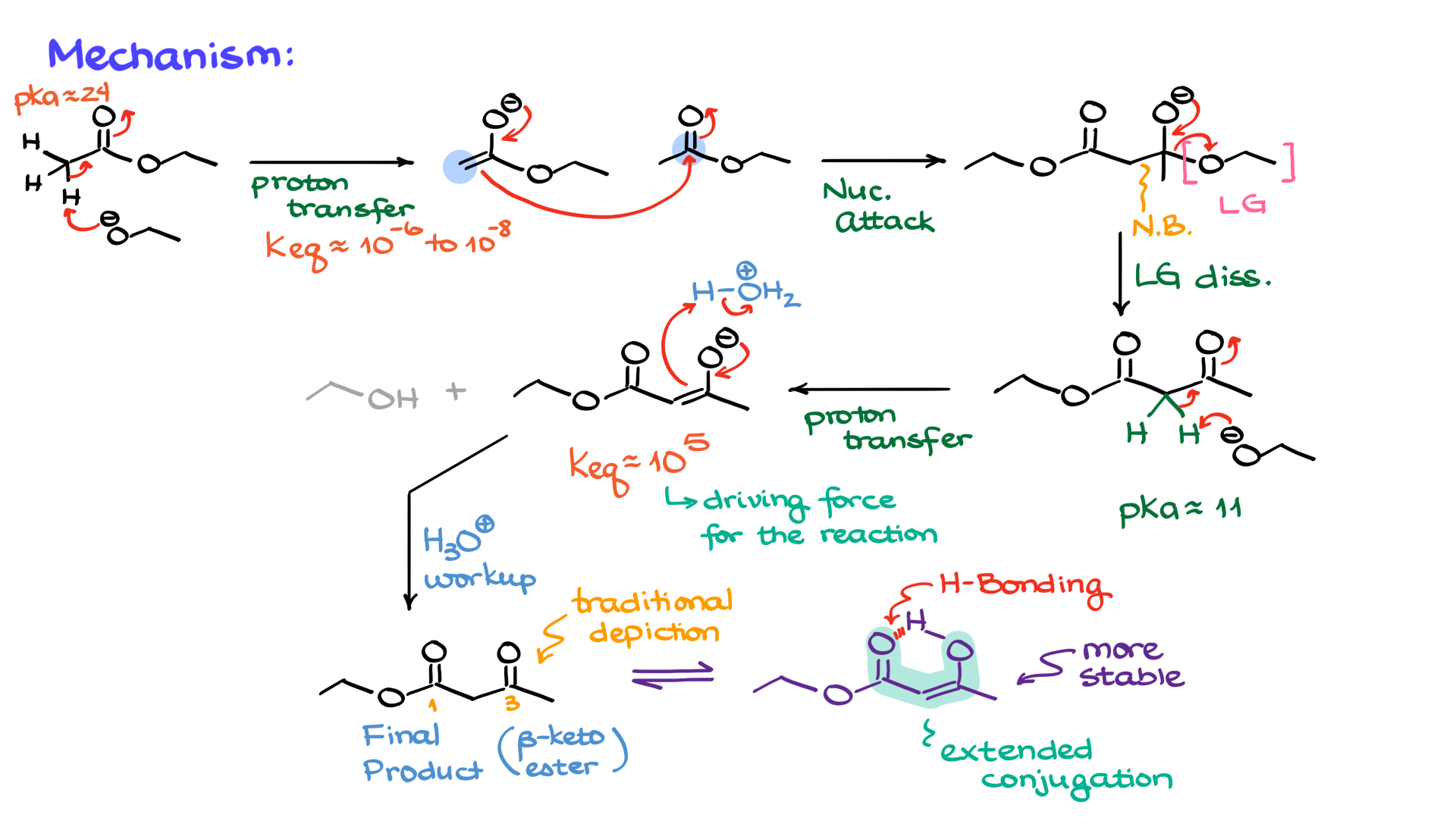
Since esters are not particularly acidic, with pKa values typically between 22 and 24, the equilibrium constant for this step is not impressive, meaning we only produce a small amount of the enolate while most of the reaction mixture remains as unreacted ester. But that works to our advantage because esters, being somewhat electrophilic, can now react with the nucleophilic enolate. In this step, a new bond forms between the alpha carbon of the enolate and the carbon of the carbonyl, using curved arrows that show the electron density flowing from the nucleophilic enolate to the electrophilic ester, forming a negatively charged tetrahedral intermediate with the newly formed bond clearly visible.
From this point, as with many reactions involving carboxylic acid derivatives, the tetrahedral intermediate loses a leaving group, which in this case is ethoxide. We label it as the leaving group and show how it is expelled from the molecule, forming what looks like the final product, albeit rotated in space compared to the earlier drawing, with the ethoxide anion floating around. But here’s the catch: the protons between the carbonyls in the product have pKa values between 9 and 13, depending on the exact nature of the molecule, making them significantly more acidic than the ethoxide just produced. This ethoxide, being too basic, immediately snatches that proton, re-enolizing the molecule and forming the corresponding enolate. This proton transfer, with an equilibrium constant around 105 to 106 depending on the molecule, is highly favorable and drives the entire reaction forward. Without this final proton transfer, the reaction would not proceed to completion, and the product would revert to the starting materials. This final proton transfer is the key to making the Claisen condensation work.
Once the enolate product is formed, an acidic workup is performed to reprotonate the molecule, eliminating the enolate and yielding the final product, a 1,3-dicarbonyl compound. In this case, the product is a 3-keto ester, also known as a beta-keto ester.
The mechanism might seem unusual because the product appears twice: once as an enolate intermediate and again as the final product after the acidic workup. The intermediate cannot be isolated because it is immediately enolized, and only after reprotonation do we obtain the final product. One more point to mention is that beta-keto esters often exist in the enol form due to intramolecular hydrogen bonding and extended conjugation, which stabilize the system. However, despite being more thermodynamically stable, these compounds are traditionally shown in their keto form, and your instructor might insist on this representation despite the enol form being more stable.
Claisen Condensation Example
Let’s look at an example using a slightly more complex ester, ethyl butyrate (or ethyl butanoate if you prefer the IUPAC name).
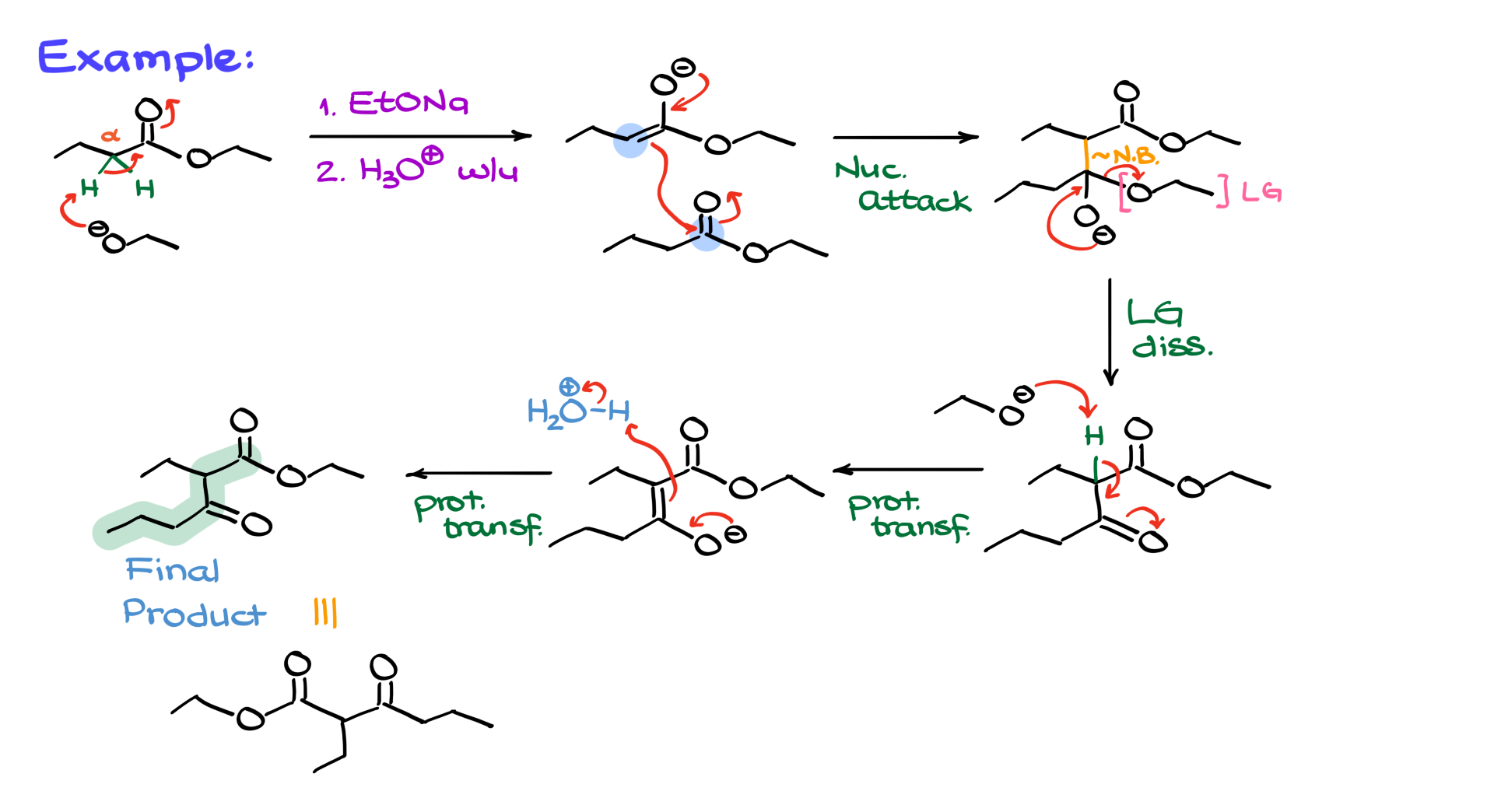
As before, we treat it with sodium ethoxide followed by an acidic workup. The alpha position next to the carbonyl is identified and deprotonated by ethoxide to form the enolate. The second equivalent of the ester is then added, and it’s helpful to draw the esters one under the other to better visualize the bond formation. The electrons flow from the nucleophilic enolate to the electrophilic ester, forming a new carbon-carbon bond between the alpha position and the carbonyl, leading to a tetrahedral intermediate highlighted with the new bond in orange for clarity. The intermediate then loses its leaving group, ethoxide, which deprotonates the proton between the carbonyls, forming an enolate. An acidic workup then reprotonates the molecule, yielding the final 1,3-dicarbonyl compound, drawn either in its linear form or more traditional representation.
Shortcut
Like aldol condensations and many other carbonyl reactions, there is a shortcut to quickly predict the product of a Claisen condensation.
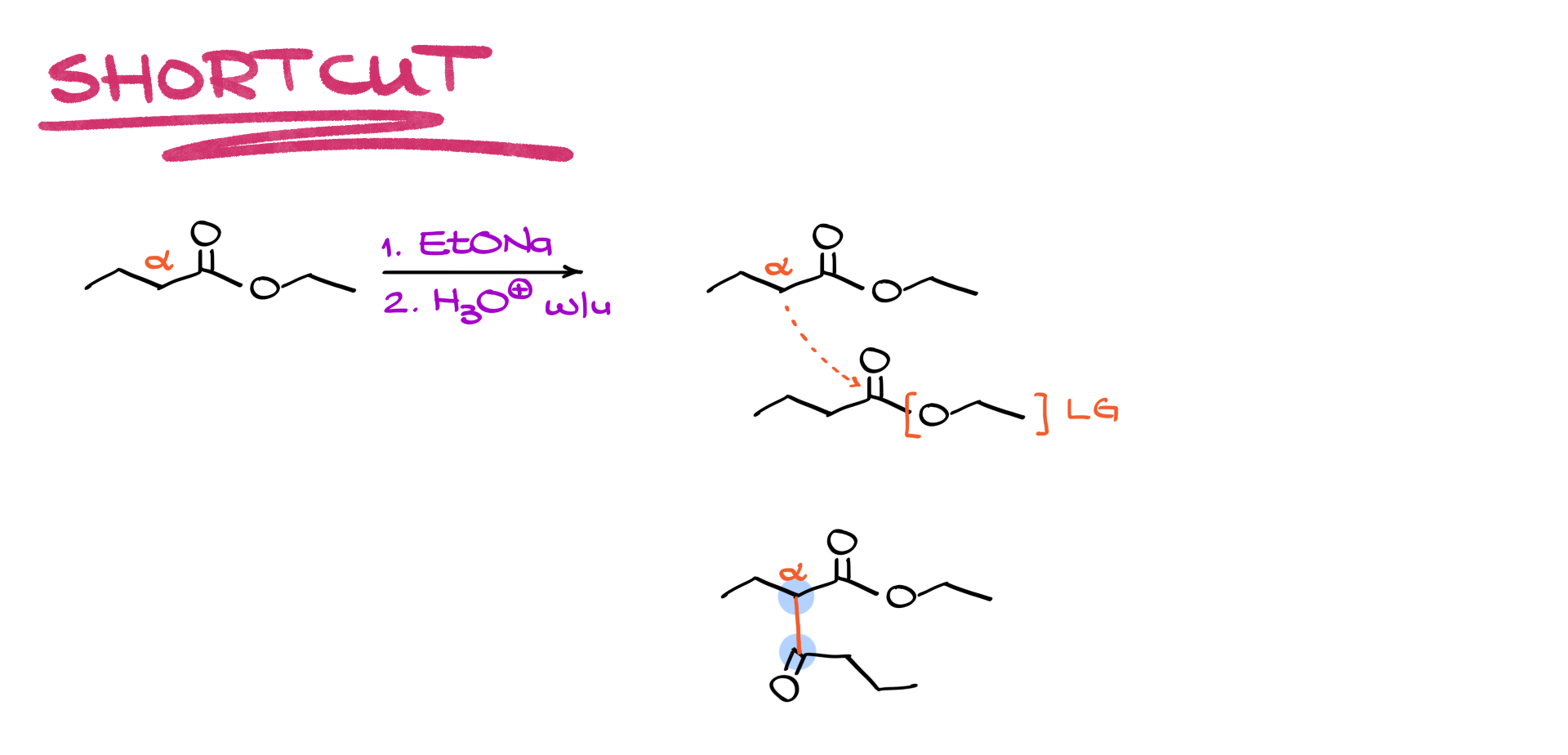
Returning to the previous example, the alpha position is identified, and the starting material is copied and redrawn underneath. A new bond is made between the alpha carbon of one molecule and the carbonyl carbon of the other, with the ethoxide leaving group removed. Rotating the molecule in space to connect the carbons gives the final product, making it a straightforward process.
Mixed Claisen Condensation
Mixed or crossed Claisen condensations add more complexity, similar to mixed aldol condensations, when two different esters are reacted.
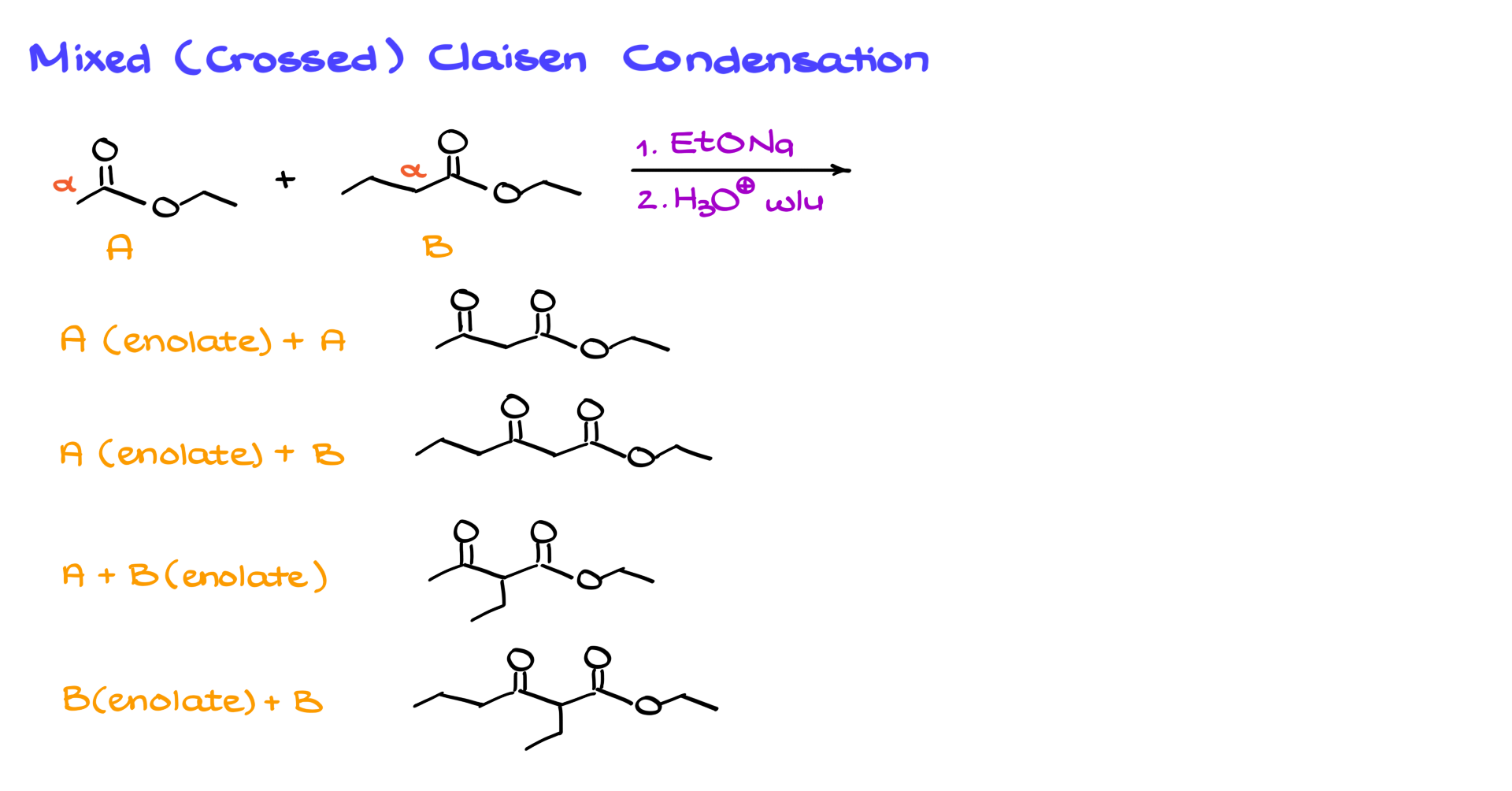
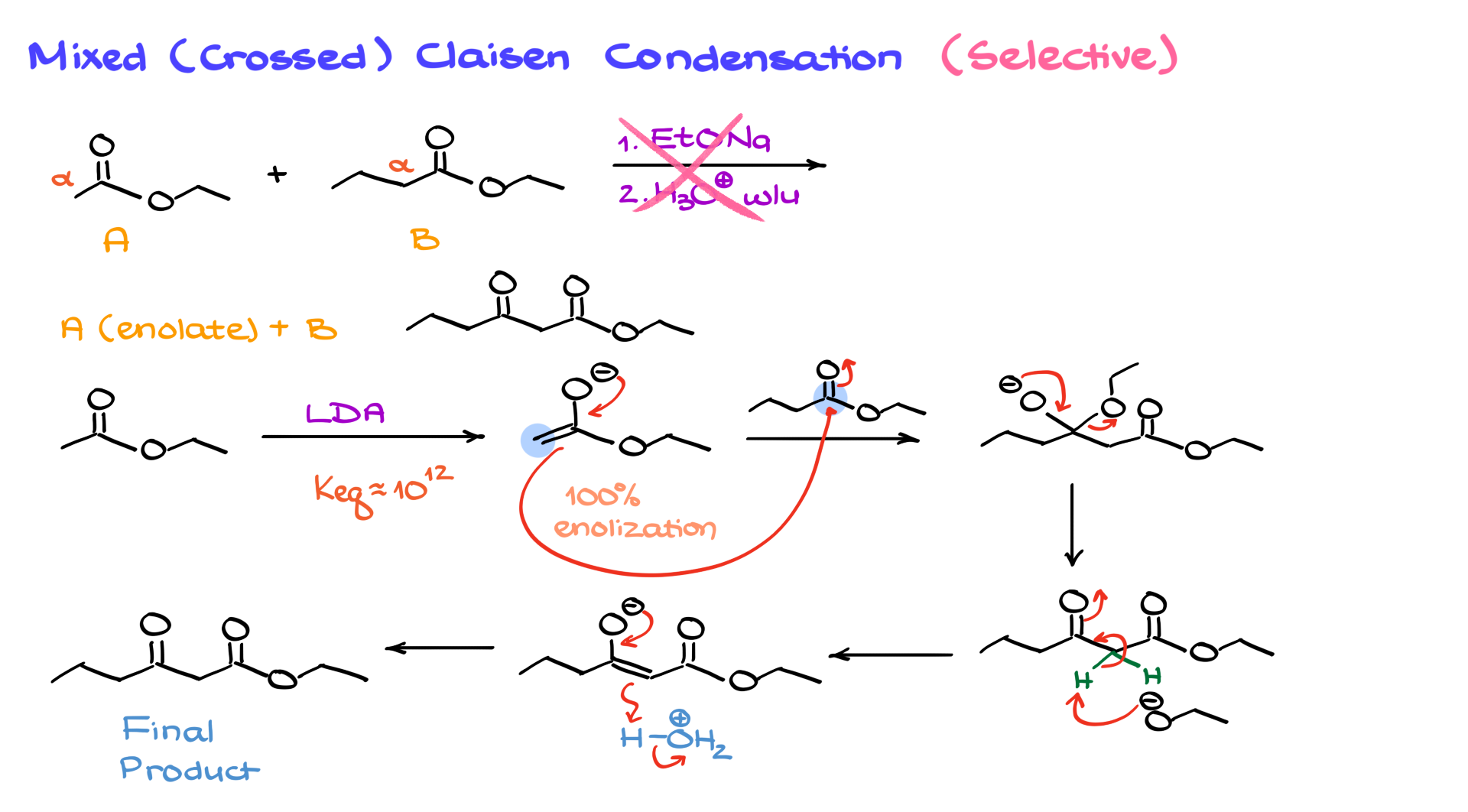
For example, combining ethyl acetate and ethyl butanoate under sodium ethoxide conditions with an acidic workup can produce four possible products due to different enolate and ester combinations, leading to a complicated mixture. To control this, selective enolization is performed using a strong base like LDA, which ensures 100% enolization of one ester. The other ester is then added to form the desired product without unwanted side reactions. The strong base ensures a high equilibrium constant for enolization, and the subsequent addition of the second ester forms the desired product after proton transfer and acidic workup.
Ketone + Ester Claisen Condensation
Another interesting application within the scope of this course is the reaction between ketones and esters in a Claisen-style condensation.
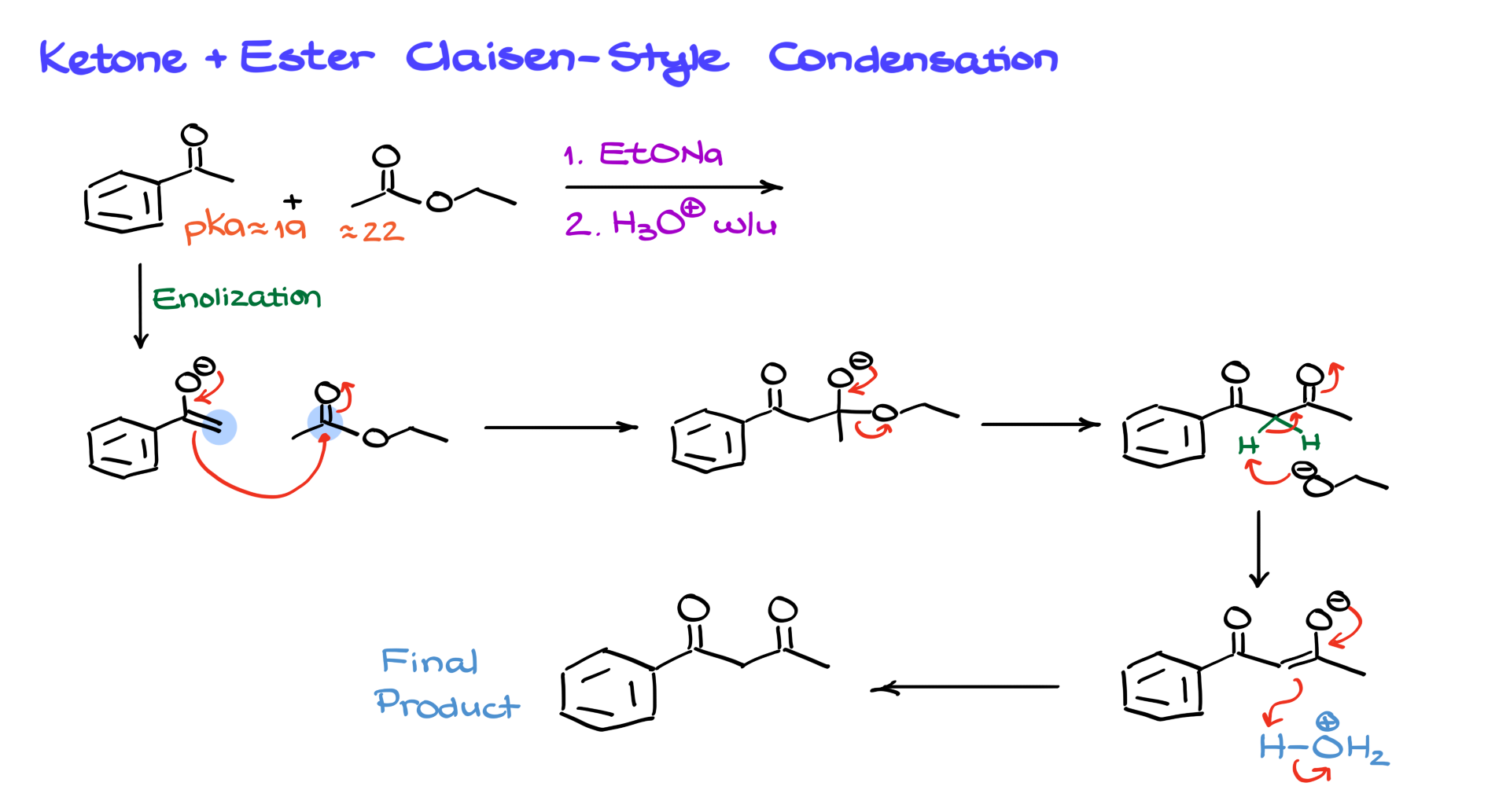
For instance, acetophenone, an aromatic ketone, reacts with ethyl acetate in sodium ethoxide solution followed by an acidic workup. Here, the ketone, with a pKa of about 19, is significantly more acidic than the ester with a pKa of 22-24, ensuring that the ketone is predominantly enolized. This enolate then reacts with the ester, forming a tetrahedral intermediate, losing the leaving group, and undergoing proton transfer to yield the final product after acidic workup.
As you can see, the Claisen condensation is a versatile and intriguing reaction with a unique mechanism. Practice the mechanism and learn to predict the products to avoid surprises during the exam.
Practice Questions
Would you like to see the answers and check your work? Become a member today or login if you’re already a member and unlock all members-only content!