Bond-Line Structures Workbook
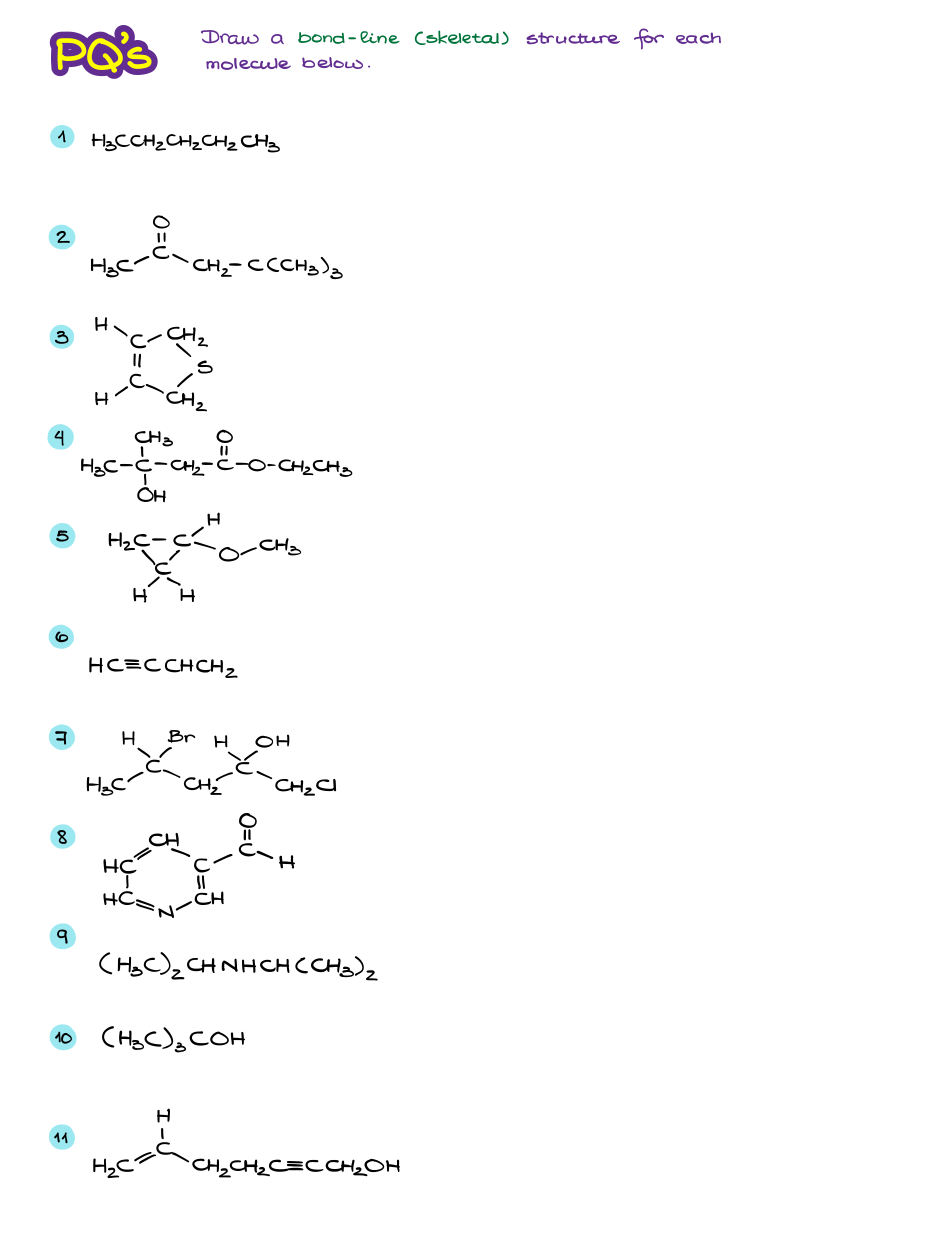
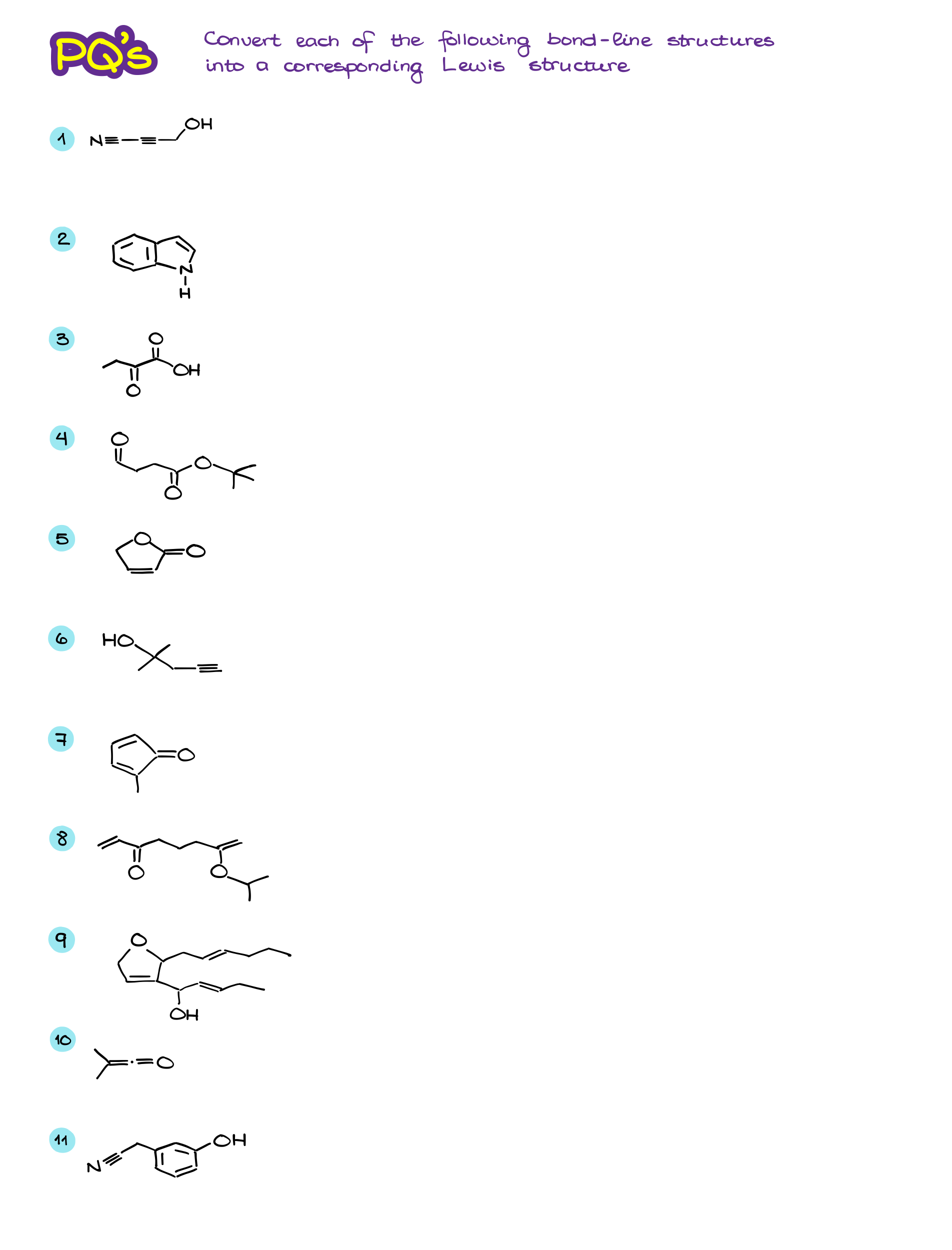
Bond-Line or “skeletal” structures are the main way of the molecular representation in organic chemistry. As I’ve already mentioned before, it’s extremely important to practice the skill of converting to and from the line structures. Many students struggle with bond-line representations simply before they didn’t do enough practice to make the skill intuitive and “automatic.” So, before you do more practice, let’s review the important points:
- We show all bonds as lines like in the Lewis structures.
- Carbons are not specified by a symbol “C” like in the Lewis structures. If there’s a line and no elements are at the end of the line (or a connection point between multiple lines)−it’s a carbon atom. We also represent chains of carbons by zig-zagging the lines with more or less realistic bond angles.
- Hydrogens on carbons are implicit. Thus, we do not indicate them by a symbol. However, hydrogen atoms on heteroatoms (anything that’s not a C) are not implicit and we must show them.
- Nonbonding electrons are also typically not represented by the dots like in the Lewis structures.
- Formal charges are not implicit and MUST be shown.
So, here’s a quick example of a few molecules and their corresponding structures.
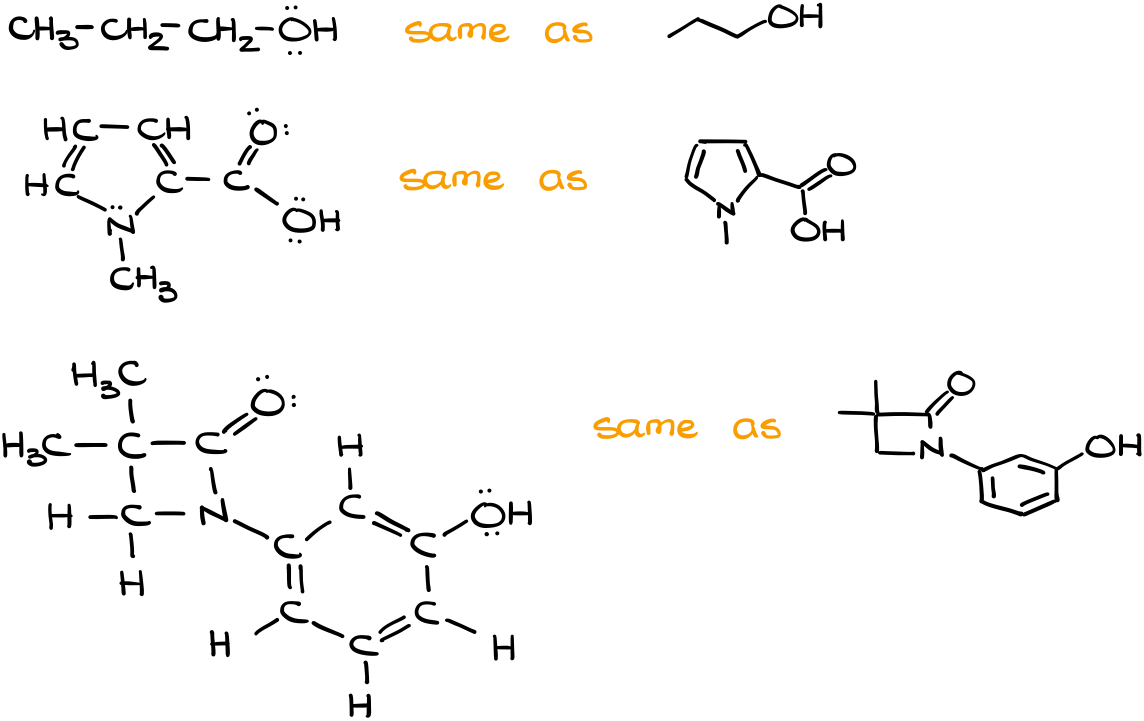
“Good Grammar” of the Bond-Line Structures
It’s also important to remember that there are “good practices” when it comes to the bond-line structures drawing. Probably the most important point is to keep the structures as close to the realistic bond angles as possible. While drawing “bad” or “exaggerated” angles won’t necessarily lose you points on the test, it would definitely annoy your instructor 😉

In other words, the cleaner your structure is, the better you’re going to be overall. As an instructor, if I have to guess what you mean by your structure, I’m going to comment about that on your homework. On the test though, I’m most likely going to take points.
Alright, now once we’ve refreshed our memory on the topic, let’s do a little practice!