Benzoin Condensation
In this post, I want to talk about an interesting reaction of aldehydes called the benzoin condensation. The reaction name originates from the name of the product you get when you perform it on benzaldehyde alone.
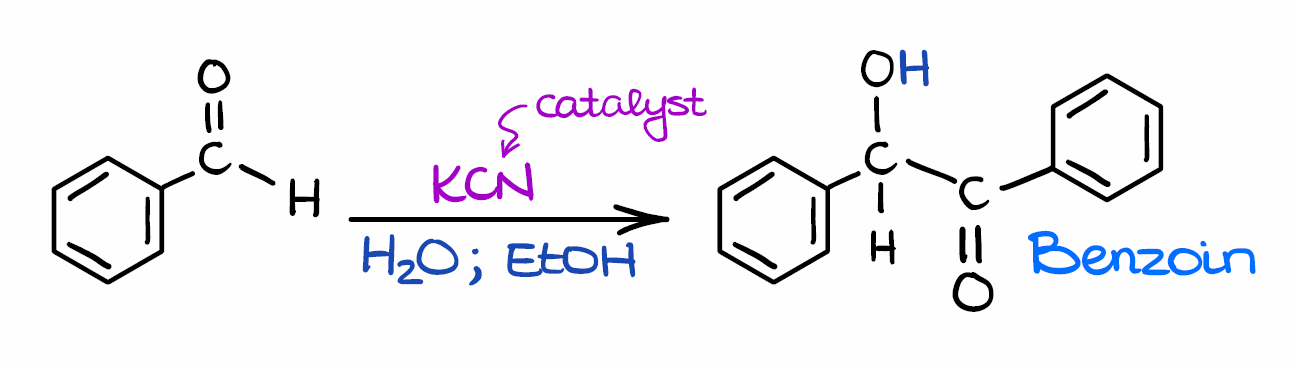
Ok, so here’s the general scheme for the benzoin condensation. Here we have a “classic” example of the benzoin condensation where the two molecules of benzaldehyde make a benzoin molecule. In this case, we are using potassium cyanide (KCN) as a catalyst. However, you may use other catalysts in this reaction as well. The two important features of the catalyst are:
- It should be somewhat nucleophilic, and
- the species needs to have electron-withdrawing properties to stabilize the intermediate.
We’ll look at the mechanism in just a few moments, so don’t worry if it doesn’t make much sense right now. I’ll specify where and why this comes into play once we get to that point. The other components of this reaction are a solvent. The nature of the solvent is only marginally important. Ideally, it should be a polar protic solvent to facilitate the proton exchange in the reaction mechanism. And it should also effectively solvate the polar particles in this reaction.
The Mechanism of the Benzoin Condensation
Step 1: Formation of a Cyanohydrin
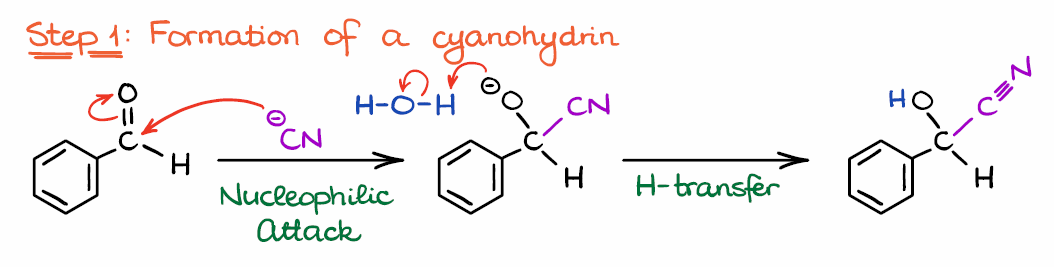
Alright, let’s look at the mechanism now. The reaction starts by the attack of the nucleophilic catalyst on the C of the electrophilic carbonyl. If you need a refresher on what is an electrophile and what is a nucleophile, I have a post on that. So, the first step here is, in a nutshell, nothing more than a formation of the cyanohydrin, which is a common reaction of aldehydes and ketones.
Step 2: Formation of a Nucleophiles from the Cyanohydrin
In the next step, we use the base formed in the first step to deprotonate the cyanohydrin and form a nucleophilic species.
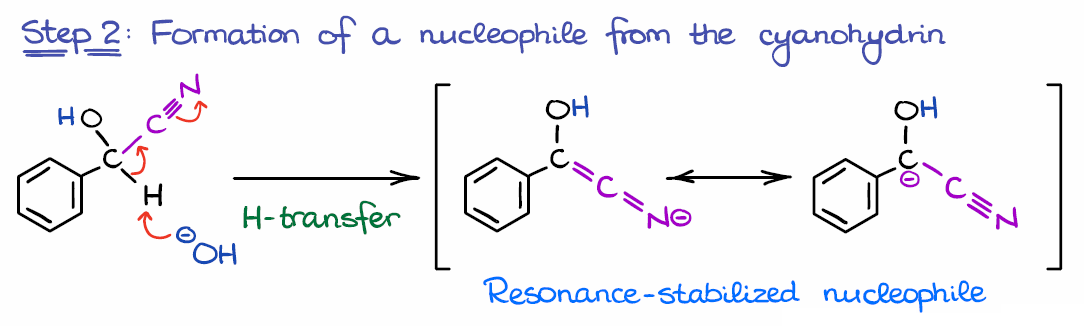
This is where the electron-withdrawing nature of the catalyst comes into play. Without cyanide (or another EWG), the proton won’t be sufficiently acidic and won’t be pulled off by a mild base like OH–.
Another important thing I want to point out for this step is the polarity reversal, or the “umpolung” of the carbonyl. Normally, we would have a significant partial positive charge (δ+) on the carbon of the carbonyl. This means that the carbon atom in the carbonyl is a typical electrophile. However, in this case, we have a negative charge on carbon, making it nucleophilic. The technique when we invert the charge on an atom in a molecule called the “umpolung” principle which literally translates from German as “inversion of polarity.”
Step 3: Reaction of the New Nucleophile with the Second Equivalent of Benzaldehyde
Now, once we have the nucleophile, we are going to react it with another molecule of the starting material—benzaldehyde in this case.
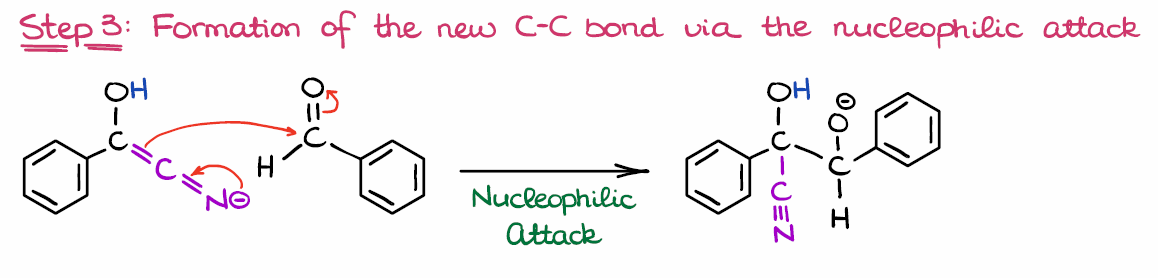
While several nucleophilic attacks are generally possible in this case, the formation of C-C bond is the most thermodynamically favorable. Thus, we are going to only look at this mechanistic step here. Since the reaction is an equilibrium, the other nucleophilic attacks will simply revert, so we don’t really need to consider those.
Step 4: Regeneration of the Catalyst
Finally, we are going to regenerate our catalyst by doing the leaving group dissociation.
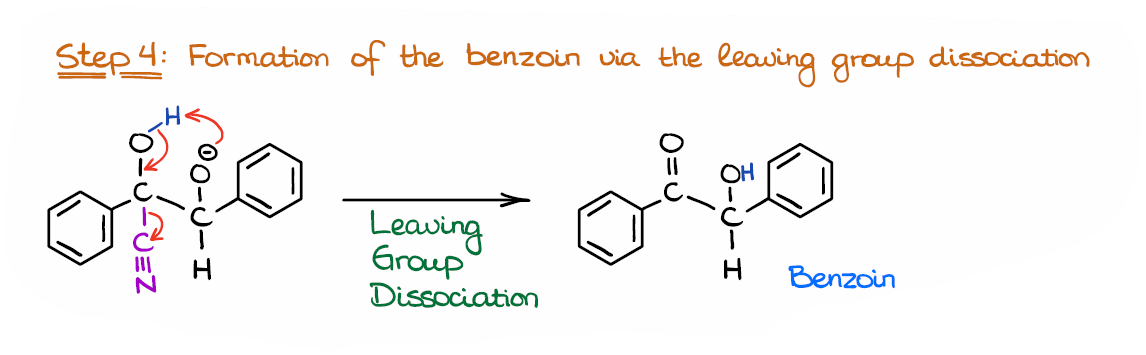
I’m technically combining two steps here doing the proton transfer between the hydroxyl and alkoxide groups and pushing the leaving group out. Whether it indeed happens in a single step or in two steps is up for a debate. At least, I couldn’t find any literature on that. So, if you know any sources I could look up, let me know in the comments below!
The Overall Mechanism and Examples of the Benzoin Condensation
Finally, we have the complete mechanism for this reaction.
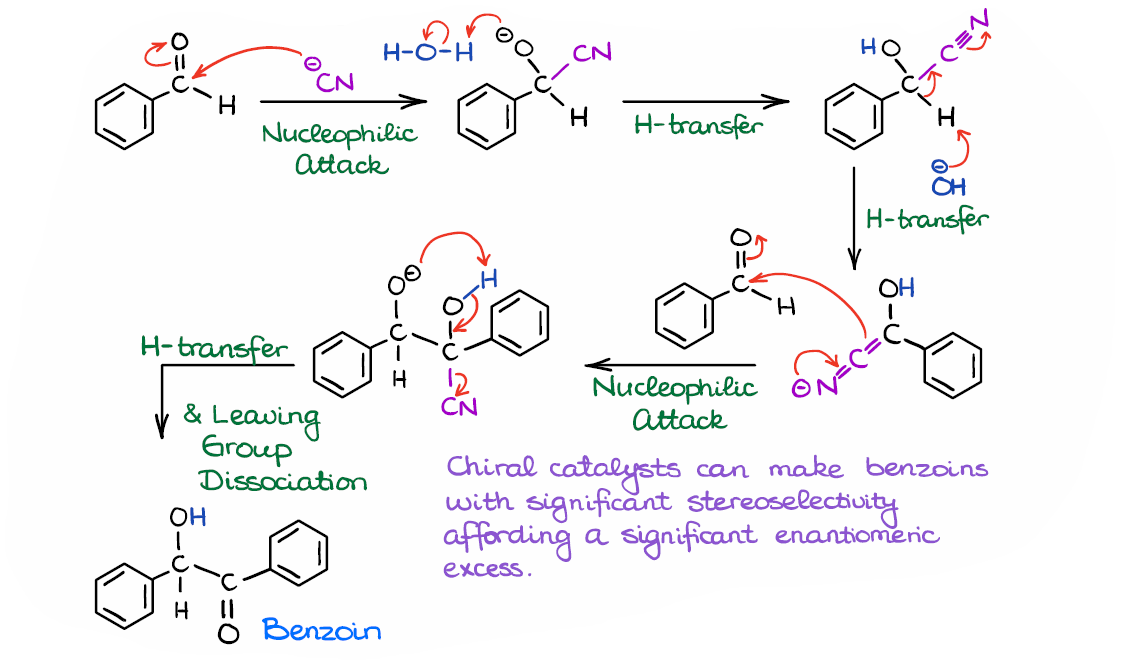
So, to do a quick recap here: we started by forming a cyanohydrin, we then deprotonated it, and reacted the resulting nucleophilic intermediate with another molecule of an aldehyde. And finally, we regenerated the catalyst by kicking it out of the molecule.
Here are a few more examples of the benzoin condensation.
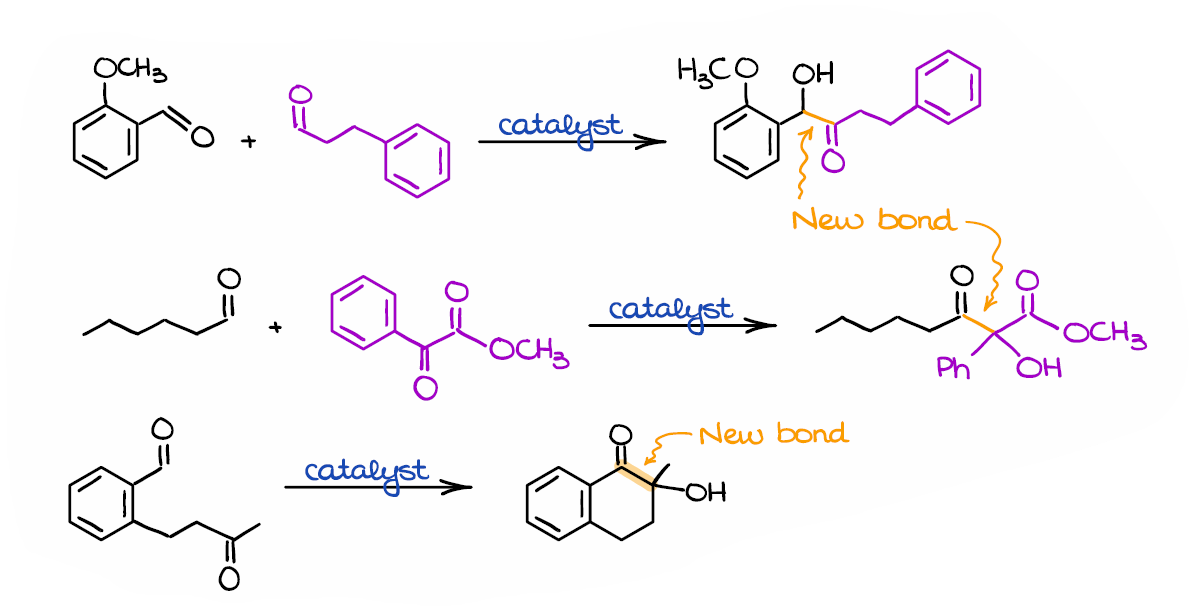
Interestingly, the benzoin condensation can be performed in a mixture of two different aldehydes, or even with an aldehyde and a ketone. The more reactive aldehyde will eventually act as a nucleophile in such mixed benzoin condensations. Likewise, if you have a mixture of an aldehyde and a ketone, it’s always the aldehyde that will eventually be a nucleophile. The benzoin condensation is also a convenient way of making cyclic molecules like in the last example here. On top of that, using chiral catalysts we can make certain stereoisomers with high stereoselectivity, which can be vitally important for medicinal chemistry and the synthesis of biologically active molecules.
Finally, I should also mention, that the benzoin condensation is an equilibrium and can be reversed. So, if you somehow manage to make an α-hydroxy carbonyl, you could “open” it via the retro-benzoin condensation. Making such α-hydroxy carbonyls is, actually, not a simple task, so I haven’t seen anyone doing the retro-benzoin condensation except for the purposes of scientific curiosity.
Anyways, hope you guys enjoyed learning about this neat little reaction. Let me know if you’ve ever heard of it before or if you’ve covered it in your course in the comments below.
I’ll see you in the next post, and until then, remember to stay awesome!
