An Unexpected Oxidation Challenge Mechanism
In this tutorial, I wanna talk about this rather fun mechanism right here.
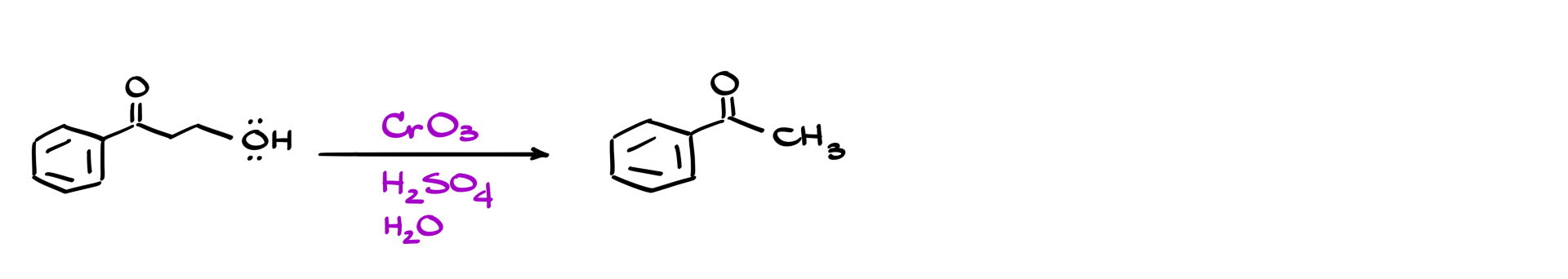
Just by looking at the reagents, it seems like we’re dealing with some kind of oxidation reaction. We’ve got a primary alcohol, and yet in the final product, we’re left with just a CH₃ group. So, clearly, we’re chopping off part of the molecule. Funny enough, when we check the oxidation states, we see the carbon is going from +2 to +3 in the product. That’s not oxidation—it’s actually a reduction. That doesn’t make any sense… or does it? Let’s dive into the mechanism and see what’s really going on.
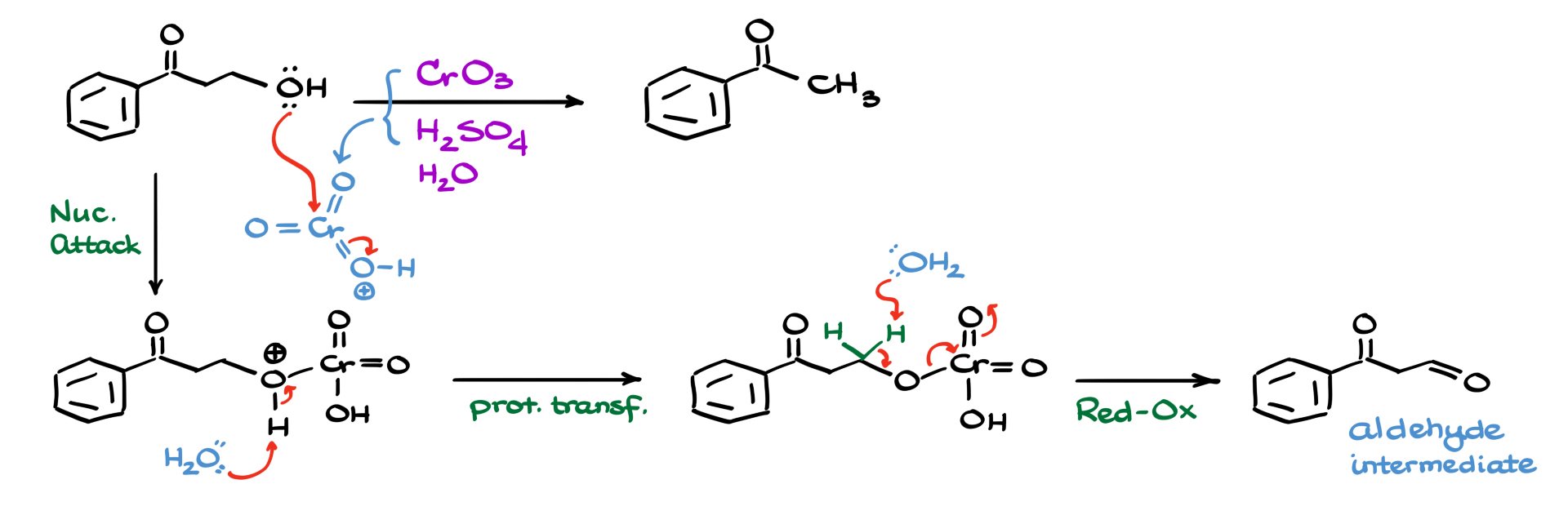
So first, I’m gonna show how chromium oxide reacts with sulfuric acid, forming a protonated, more electrophilic species. From there, the oxygen on our alcohol attacks the chromium center, giving us the first intermediate. Since we’re in aqueous conditions, water comes in and pulls off that proton, giving us a neutral intermediate where the chromate is now attached to our molecule.
Now, because this is a typical Jones oxidation, I’m going to show the key redox step. Water acts as our base and pulls off a proton, setting off the electron shifts that lead us to our aldehyde intermediate. But since we’re in acidic aqueous conditions, the aldehyde doesn’t just sit around. Instead, it reacts further—we move straight into hydrate formation.
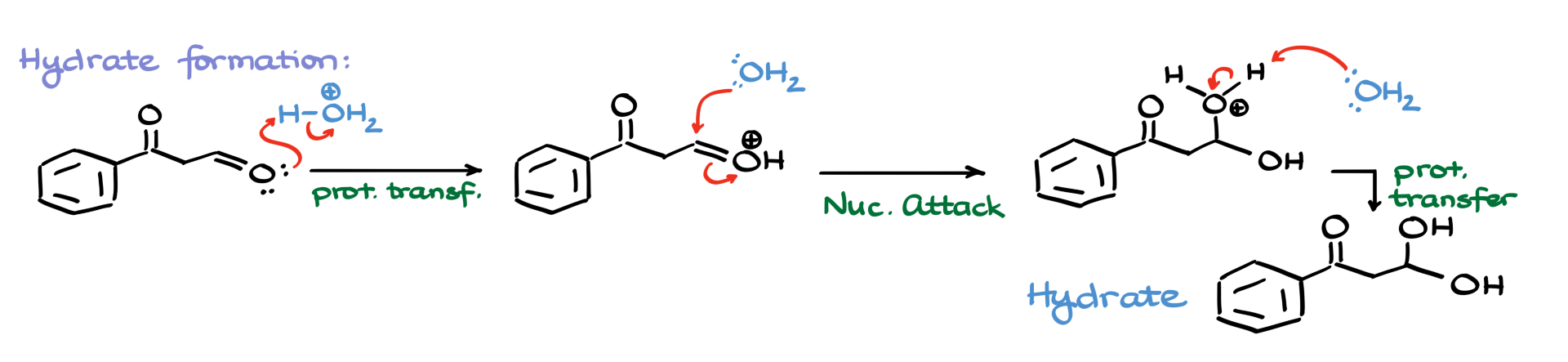
So I’ll redraw the aldehyde, and under acidic conditions, it gets protonated by our acid. This protonated intermediate then reacts with water, which attacks the carbon, forming another intermediate. We neutralize this by using another water molecule to pull off the extra proton, and just like that, we’ve got our hydrate.
But that’s only the halfway point in this reaction. Jones oxidation doesn’t stop there. Let’s go through the second round. I’ll redraw the hydrate, bring in more chromium oxide, and just like before, the alcohol portion attacks the chromium center. We add water again to deprotonate the intermediate, giving us another neutral species ready for oxidation. Water comes in again, pulls off another proton, shifts the electrons, and boom—we’ve now got our carboxylic acid.
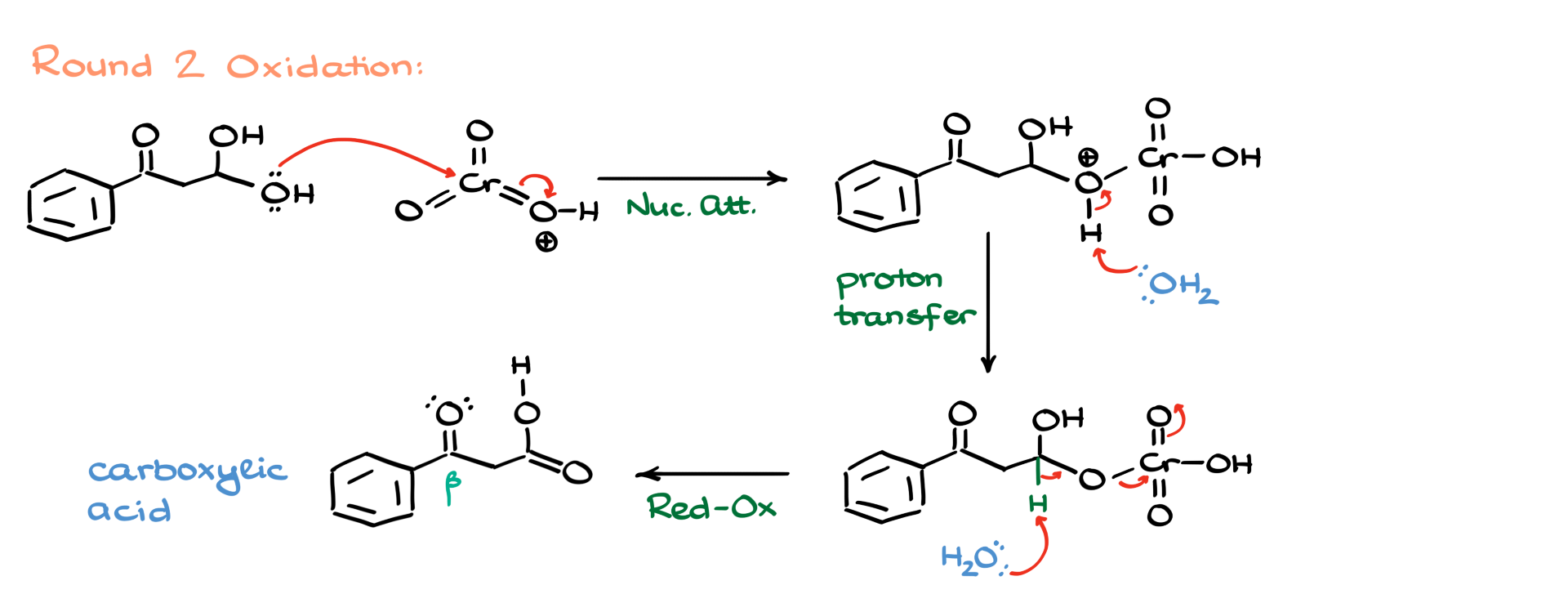
Here’s where it gets interesting. This particular carboxylic acid is a β-keto carboxylic acid, which aren’t exactly stable. These compounds tend to decompose, so that’s what happens next. I’ll show the oxygen grabbing a proton, triggering a cascade of electrons that leads to the formation of an enol intermediate.
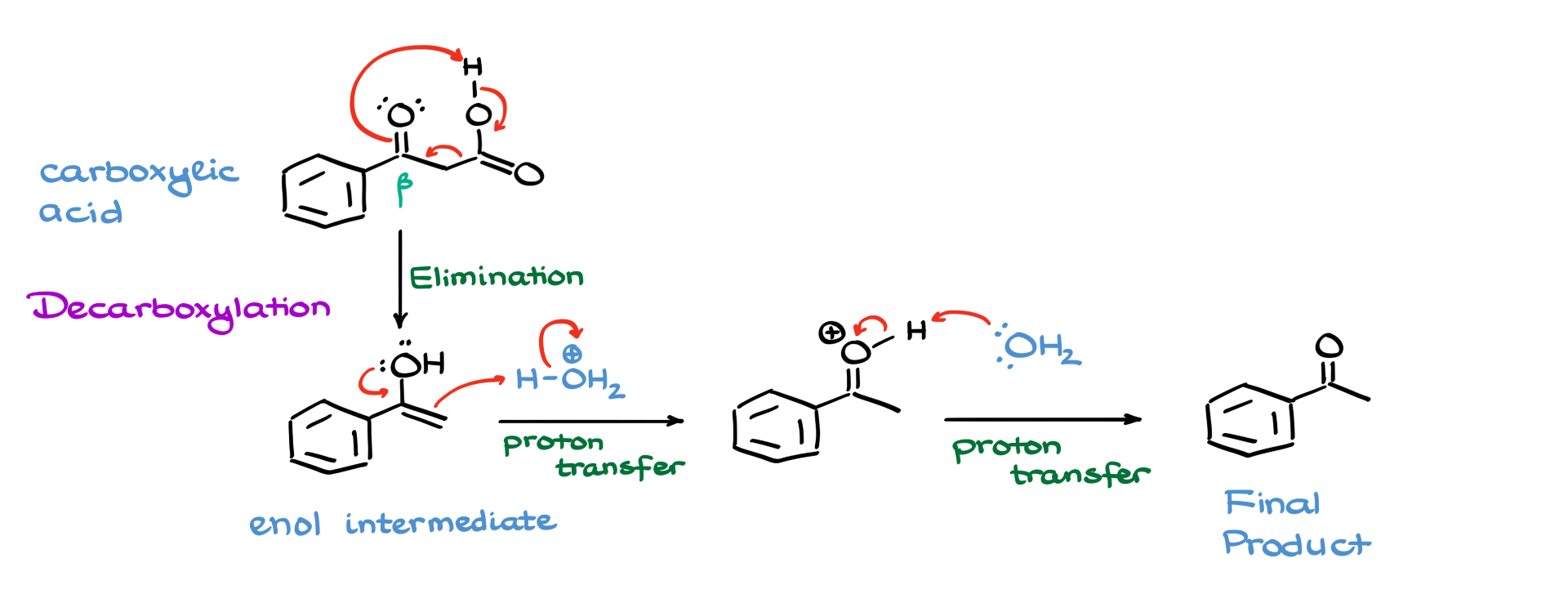
Now enols, as we know, are pretty unstable too. So this one’s going to go through keto-enol tautomerization under acidic conditions. I’ll show my enol picking up a proton from the acid, forming a protonated intermediate. Then we use another water molecule to pull that proton off, finally giving us our stable end product.
So as you can see, this started out as a straightforward Jones oxidation, but because of the nature of the intermediate and the final structure we were heading toward, the reaction went even further. That’s a really important point—whenever you think you’ve reached your final product in any reaction, always stop and ask: “Can anything else happen to this molecule?” Because if it can, it probably will!
